Why Fe, Co and Ni shows ferromagnetic properties
Q.Why Fe, Co and Ni shows ferromagnetic properties ?
Generally , the magnetic properties of elements arises due
to the present of unpaired electros in its outer most valence shell.
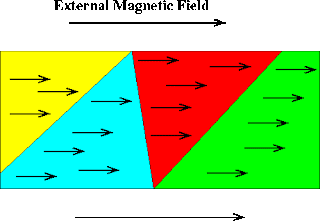
In case of Fe,Co and Ni , the magnetic moment due to unpaired electron spins are aligned parallel to
the external magnetic field more efficiently resulting in an exceptionally strong reinforcement of paramagnetism.
These substances are therefore, much more paramagnetic than the rest of the elements ,are said to be ferromagnetic.
In case of Fe,Co and Ni , the magnetic moment due to unpaired electron spins are aligned parallel to
the external magnetic field more efficiently resulting in an exceptionally strong reinforcement of paramagnetism.
These substances are therefore, much more paramagnetic than the rest of the elements ,are said to be ferromagnetic.







No comments