shielding effect Slater's rules and its limitations.
What is shielding effect ?
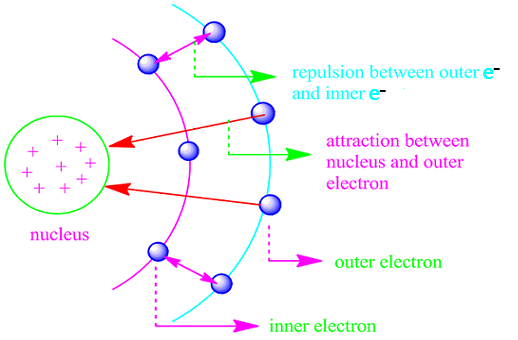
In multi-electron atoms, there are a number of electrons .Some of them,occupying lower energy orbitals, tend to reduced the effect of nuclear charge on the electrons present in higher energy orbitals.
This is due to the fact that these orbitals intervene between the nucleus and the electrons in the higher energy orbitals.This effect is known as shielding effect .
What is effective nuclear charge ?
An electron in one of the atomic orbitals of a multi-electron atom felt electrostatic repulsion by other electrons in the same or other orbitals.
As a result of this, the nuclear charge felt by this electron will be less than the actual nuclear charge.
This electron is said to be screened from the influence of the nuclear charge and the reduced charge felt by the electron is known as the effective nuclear charge.
Zeff =Z actual – s
where 's' is the shielding constant whose magnitude determines the extent to which the electron shielded by other electrons in the atom from the nuclear charge.
What is Slater rules ?
Slater has given a set of empirical rules for calculating the shielding constant for an electron is known as Slater rules. These rules are,
Case-I. For S
or P electrons.
1.The electronic configuration should be in the following
order and grouping.
(1s) ( 2s 2p) ( 3s 3p) ( 3d ) ( 4s 4p ) (4d ) (4f) (5s 5p) .
2.Electrons in any group to the right of the ( ns
np ) group contribute nothing to the shielding constant.
3.( a) All other electrons in the ( ns np ) group shields the valence electron to an extent of 0.35 each.
(b) Contribution is 0.30 if the group is 1s.
4.All electrons in the ( n- 1) shell shield to an extent of 0.85 each.
5.All the electrons in ( n – 2) shell or lower shells shield completely ,that is contribute 1.00 each.
Case-II. For d or f –electrons.
Rules (i), (ii) 3(a)
remains same 3(b),4 and 5 are replaced by rule 6
Application of Slater rules.
The main application of Slater rules are calculating of shielding constant for an electron in atom.Other application of Slater rules.
Why 4s orbital is filled earlier than a 3d orbital ?
The 19th electron of an atom may enter a 4s or a
3d orbital. But a 4s electron would be subjected to a greater electrostatic
attraction due to a higher nuclear charge than a 3d electron.
Consequently, the atom with outer electronic configuration 3s2 p6 4s1 having lower energy is more stable than with outer electronic configuration 3s2p6 3d1 having higher energy.
Consequently, the atom with outer electronic configuration 3s2 p6 4s1 having lower energy is more stable than with outer electronic configuration 3s2p6 3d1 having higher energy.
Thus, a 4s orbital is filled earlier than a 3d orbital.
Why the transition metal atoms lose ns electrons first than 3d during ionization?
In transition metal atoms, the calculated value of effective nuclear charge felt by the 3d electron
is 5.60 where as the
effective nuclear charge felt by the 4s electron is 3.6.
Evidently, the attraction between the 4s electrons and the nucleus is less than the attraction between the 3d electrons and the nucleus.
Hence, the removal of the 4s electrons from an isolated gaseous atom would be easier than the removal of the 3d electron .
For this reason, the transition metal atoms lose ns electrons first than 3d during ionization.
Why the first ionization energy is always lower than second and third ionization energy ?
The first ionization energy is always lower than the second
ionization energy and second ionization energy is always lower than third
ionization energy and so on .
This is because the effective nuclear charge goes on increasing with each removal of electron from an atom.
This is because the effective nuclear charge goes on increasing with each removal of electron from an atom.
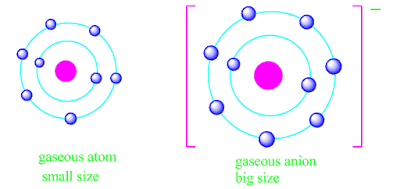
Why an anion is always bigger and a cation is always smaller than the parent atom ?
The calculated value of effective nuclear charge of an anion is less than the concern atom .
Therefore, the electrostatic attraction between the electrons and the nucleus is more in neutral atom than anion.
As a result, the nucleus would have lesser hold on electrons in the anion than in the neutral atom.
Consequently, the size of anion is always larger than the size of neutral atom.
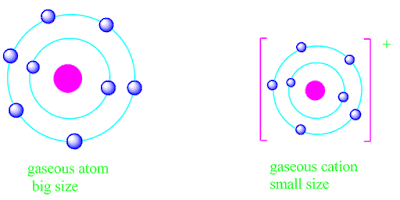
Similarly, the calculated value of effective nuclear charge
of neutral atom is less than the cation of that atom.
Therefore, the hold of the nucleus on the electrons is less in the case of neutral atom than in case of the cation.
Consequently, cation is always smaller than neutral atom.
Therefore, the hold of the nucleus on the electrons is less in the case of neutral atom than in case of the cation.
Consequently, cation is always smaller than neutral atom.
Limitations of Slater’s rules .
The Slater rules have some limitations which are discussed below.
1. According to Slater’s rules, the outer shell electrons do
not shield the inner electrons .
This may not be true. For example, even the outer S electrons penetrate quite to the nucleus and would thus exercise a shielding effect.
This may not be true. For example, even the outer S electrons penetrate quite to the nucleus and would thus exercise a shielding effect.
2. We know that s-electrons are more penetrating than p-electrons of the same shell so that the screening by the 's' electrons will be more than the screening by the p'-electrons of the same shell is the same.
3. According to the Slater’s rules, the s,p,d and f electrons of the same shell screen the higher shell electrons to the same extent.
This is, however , not correct. The electrons in s,p,d and f orbitals differ so much in their penetrating power.
An ns electrons has greater probability of being present near the nucleus than an np electron.
Similarly, an np electron has greater probability of being present near the nucleus than an nd electron.
The electron present in these orbitals would not screen the outer shell electrons to the same extent.
What is shielding effect ?
What is Slater's rules ?
What is the application of Slater's rules ?
What is the application of Slater's rules ?
What is the limitation of Slater's rules ?
Why 4s orbital is filled earlier than a 3d orbital ?
What is effective nuclear charge ?








Nice website for learning 😃😃
ReplyDeleteNice blog
ReplyDelete