What is Borodine Hunsdiecker reaction and its limitation ?
What is Borodine Hunsdiecker reaction ?
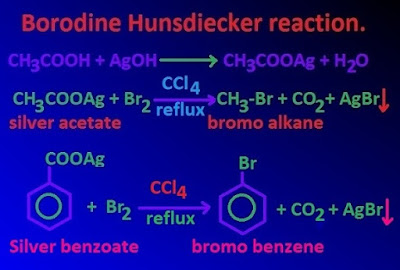
When the silver salt of carboxylic acid is treated with bromine molecule in the presence of CCl4 solution , bromo alkane or alkyl bromide is obtained . Then the reaction is known as Borodine Hunsdiecker reaction .
In Borodine Hunsdiecker reaction, the obtaining alkyl bromide content one carbon less than the parent silver salt of carboxylic acid .
In this reaction the amount of extent of the alkyl bromide depends on the nature of alkyl group .
So, the
order of obtaining alkyl bromide should be primary > secondary > tertiary.
In Borodine
Hunsdiecker reaction, chloro alkane may be also prepared , but the amount of
extent of product ( chloro alkane ) is very much less .
Mechanism of Borodine Hunsdiecker reaction: The mechanism of the Borodine Hunsdiecker reaction are shown below,
Application of Borodine Hunsdiecker reaction .
Borodine Hunsdiecker reaction is used to prepare mainly alkyl bromide and alkyl chloride from the silver salt of carboxylic acid . Besides, this reaction is applied to decrease the carbon chain .
Limitation of Borodine Hunsdiecker reaction.
In this method alkyl iodide can’t be prepared , because , when iodine is treated with the silver salt of carboxylic acid , in stead of alkyl iodide , ester is the product.
This reaction is known as Birnbaun –Simonini reaction .
Why the neopentyl chloride can’t prepared by the reaction of neopentyl alcohol and HCl ?
The substrate neopentyl alcohol does not takes part in SN2 reaction mechanism due to steric crowding of methyl groups.
Again, with HCl acid neopentyl alcohol forms primary or 1ᵒ carbocation ,which is very much unstable .
So this intermediate carbocation being unstable , soon it under goes rearrangement reaction.
That is, by the shifting of methyl group it convert into tertiary carbocation which is very much stable than primary carbocation .
In the second step , the nucleophile Cl– attack the carbocation , resulting in the formation of 2-chloro-2-methyl butane .
Consequentlt, neopentyl chloride can’t prepared by the reaction of neopentyl alcohol and HCl .
What is Borodine Hunsdiecker reaction ?
Application of Borodine Hunsdiecker reaction.
Limitation of Borodine Hunsdiecker reaction. .
Why neopentyl chloride can’t prepared by the reaction of neopentyl alcohol and HCl .










No comments