Benzyl alcohol to benzoic acid change-Schmidt reaction-HVZ reaction.
Benzyl alcohol to benzoic acid change
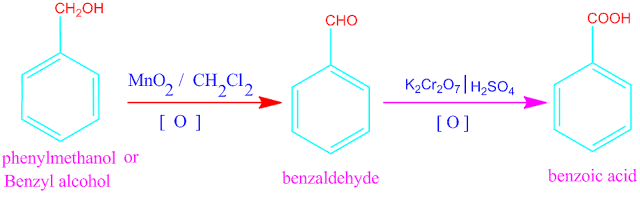
Benzyl alcohol is a aromatic primary alcohol with molecular formula C6H5CH2OH. It is colorless liquid with a mild pleasant aromatic odor.
Benzoic acid
may be prepared from benzyl alcohol easily. The change of benzyl alcohol to
benzoic acid takes two step.
In first step, benzyl alcohol is converted into benzaldehyde through the oxidation reaction by active MnO2 in presence of inert solvent , CCl4 or CH2Cl2 .
In second
step, further oxidation of this benzaldehyde by potassium dichromate and
sulfuric acid, gives benzoic acid .
Benzoic acid to benzyl alcohol change.
Benzoic acid
can be change into benzyl alcohol through the reduction reaction by lithium
aluminium hydride .
What is HVZ or Hell-Volhard-Zelinsky reaction?
If aromatic or aliphatic carboxylic acid with alpha-carbon that is,with alpha-hydrogen
is treated with chlorine or bromine in
presence of a little amount of red phosphorous catalyst, the product obtained is alpha-chloro or alpha-bromo acid.
This substitution reaction is known as HVZ or
Hell-Volhard-Zelinsky reaction. In this
reaction , alpha-hydrogen is substituted by chlorine or bromine atom.
The carboxylic acid which participate in HVZ or Hell-Volhard-Zelinsky reaction, must be contain at least one alpha-hydrogen atom.
If excess
amount of halogen is used in this reaction, then more one alpha-hydrogen atom
is substituted by halogen atom.
Why does benzoic acid not exhibit HVZ reaction?
The carboxylic acid which participate in HVZ or Hell-Volhard-Zelinsky reaction , must be contain at least one alpha-hydrogen atom.
Although, benzoic acid is a carboxylic acid, yet it does not exhibit HVZ reaction. Because , benzoic acid has no alpha-hydrogen atom.
In the same
reason , formic acid does not exhibit HVZ reaction, that is, formic acid has no alpha-hydrogen atom.
What is Schmidt reaction ?
If aromatic or aliphatic carboxylic acid is reacts with hydrazoic acid in presence of concentrated sulfuric acid, primary amine is obtained. This reaction is known as Schmidt reaction.
In this
reaction, the obtaining primary amine have one carbon less than the parent
carboxylic acid.
Summary
- Benzyl alcohol to benzoic acid change
- Benzoic acid to benzyl alcohol change .
- Why benzoic acid does not exhibit HVZ reaction ?
- What is Schmidt reaction ?
- What is HVZ or Hell-Volhard-Zelinsky reaction ?












No comments