Lanthanide contraction-definition-causes-consequences in chemistry
Lanthanide contraction definition in chemistry
In case of lanthanide elements , it has been experimentally found that with increasing atomic number , the atomic and ionic size of lanthanide elements gradually decreases from lanthanum to lutetium.
It is very
much regular for lanthanide ions ( Ln 3+) . It will be seen that on
going from La 3+ to Lu 3+, the ionic radius shrinks
from 115 pm to 93 pm .
The steady decrease in ionic radius all along the
series amounting in all to 22 pm , is called lanthanide contraction .
Lanthanide contraction causes
In case of lanthanide series elements, the differentiating electron entered into the 4f-orbital one at each step .
Now, the shielding or screening effect of
‘f’-orbital is very much less than ‘s’ , ‘p’ even than that of ‘d’-orbital .
This is due to the shape of ‘f’-orbital.
Hence the
effective nuclear charge increases by one at each step . Consequently , the
inward pull experienced by the 4f electrons increases.
As a result
, the size of the entire 4f n shell reduces . The sum of the
successive reductions gives the
lanthanide contraction .
Lanthanide contraction consequences
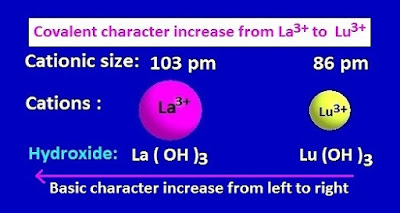
( I ) Change of basic properties of lanthanide metal hydroxide compounds .
Due to
lanthanide contraction , the ionic size from La 3+ to Lu 3+ along lanthanide
series gradually decreases.
As a result,
the covalent properties of lanthanide metal hydroxide compounds regularly increases [according to Fajan’s
rule,]from lanthanum to lutetium .
That is ,Lu
( OH )3 is more covalent than La ( OH )3 . Now , with
increasing covalent character, their basic property decreases .
Consequently, the most basic hydroxide among the lanthanide elements is La ( OH )3 and the least basic hydroxide is Lu ( OH )3 .
( II ) The
properties of metal ions are determined by their size and charge .Since all
the lanthanide ions are of about the same size and also carry the same charge due to lanthanide contraction, their
properties almost identical.
Hence
separation of lanthanides from one another is very much difficult.
( III) The
difference of density between the second and third transition metal series .
The density of second and third transition
metal series are much more higher due to lanthanide contraction .
Because the
atomic volume of two different elements of 4d and 5d series with same group is
near about same , but atomic mass of 5d series elements becomes much higher.
( IV )The
decrease in chemical reactivity of next transition elements on the lanthanide .
Due to
lanthanide contraction, the ionization energy of the next on the lanthanide
elements increases. Hence , their chemical reactivity decreases.
For
examples, the chemical reactivity of the element which belong to sixth period
such as, gold ( Au ), platinum ( Pt ), mercury ( Hg ) etc are very much less.
( V )
Similarities in atomic size of second and third transition series elements .
Due to
lanthanide contraction, the atomic and ionic radius of the next and before on
the lanthanide elements with same group,
are about same.
For examples, the atomic radius of Zr [ group – 4 , period – 5] and Hf [ group – 4 , period – 6] are 160 pm and 159 pm respectively
.
( VI )
Separation difficulties between second and third transition series elements .
The atomic
and ionic radius of second and third transition series elements are identical
to each other due to lanthanide contraction.
As a result,
their physical and chemical properties are similar to each other . Hence ,
separation of second and third transition series elements from each other are
much difficult.
For example,
separation of ‘ Zr’ from ‘Hf’ is very much difficult due to lanthanide contraction.
Why is La ( OH )3 more basic than Lu ( OH )3 ?
It has been experimentally found that with increasing atomic number , the atomic and ionic size of lanthanide elements gradually decreases from lanthanum to lutetium due to lanthanide contraction.
As a result, the covalent properties of lanthanide metal hydroxide compounds regularly increases [according to Fajan’s rule,]from lanthanum to lutetium .
That is ,Lu ( OH )3 is more covalent than La ( OH )3 . Now , with increasing covalent character, their basic property decreases .Consequently, La ( OH )3 is more basic than Lu ( OH )3 .
Lanthanide contraction consequences
Why is La ( OH )3 more basic than Lu ( OH )3 ?










Nice blog and valuable for all people. Thank you for posting this.
ReplyDeleteIonic Training in Chennai
Ionic 2 training
IELTS Training in Chennai
Japanese Language Course in Chennai
TOEFL Training in Chennai
IELTS course in chennai
spoken english course in chennai
French Language Classes in Chennai
spanish courses in chennai
content writing course in chennai
Ionic Training near me
Ionic Training in Velachery
Good content. You write beautiful things.
ReplyDeletevbet
mrbahis
hacklink
vbet
taksi
sportsbet
sportsbet
hacklink
mrbahis
Good text Write good content success. Thank you
ReplyDeletetipobet
betmatik
kibris bahis siteleri
slot siteleri
bonus veren siteler
mobil ödeme bahis
betpark
poker siteleri
thanks for sharing this is very helpful
ReplyDeletebetmatik
kralbet
betpark
tipobet
slot siteleri
kibris bahis siteleri
poker siteleri
bonus veren siteler
mobil ödeme bahis
O3AZ
elf bar
ReplyDeletebinance hesap açma
sms onay
C4QNXV
çeşme
ReplyDeletemardin
başakşehir
bitlis
edremit
Y2E4GQ
manisa
ReplyDeletetunceli
amasya
balıkesir
şırnak
56XY
https://saglamproxy.com
ReplyDeletemetin2 proxy
proxy satın al
knight online proxy
mobil proxy satın al
KQ55B
<a href="ht
ReplyDeleteشركة مكافحة حشرات بالجبيل tyUVPrwc4C
ReplyDeleteعزل أسطح القطيف
ReplyDeletePtBSl8p6