Important description of HCl acid including properties-preparation-application
Important description of HCl acid in chemistry
HCl
acid is also known as muriatic acid, is a chemical compound of
hydrogen and chlorine with molecular formula, HCl.
It is polar covalent
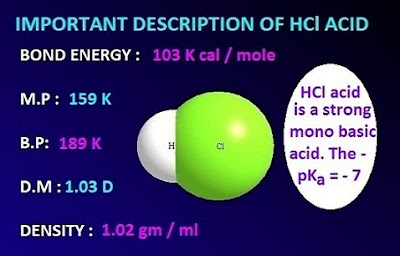
compound. The dipole moment of H–Cl
acid is 1.03 Dbye.
The another name of HCl acid are hydrogen chloride, chlorane,
spirits of salt, hydronium chloride etc.
HCl acid is a strong mono basic weak acid. The pKa value ofHCl acid is –7. The 100 ml concentrated HCl acid solution contains 39 ml of
pure HCl.
The calculated density of concentrated HCl acid is 1.2
g/c.c. Although, HCl acid
is a covalent hydride of hydrogen yet it has some ionic character due to high
electronegativity of chlorine atom.
Important properties HCl acid in chemistry
Physical
state
HCl acid is a gas at
room temperature. Because HCl acid can’t form hydrogen bond due to less
polarity of H – Cl bond.
But it is easily compressed to colorless liquid under low
temperature and high pressure. The liquid form of HCl gas is known as HCl acid.
Melting and boiling point of HCl acid
The melting
and boiling point of HCl acid are, –114ᵒC
and –84ᵒC respectively. Due
to less polarity of H–Cl bond, HCl acid does not form inter molecular hydrogen
bonding.
That
is, HCl molecules remain as an individual molecule. Consequently, the melting point and
boiling point of HCl acid is less than HBr and HI acids.
Bond dissociation energy and stability
Chlorine
is a strong electronegative element than hydrogen. So, H–Cl bond is more polar
and more strong than H–Br and H–I bond.
Hence,
the bond dissociation energy of H–Cl bond is higher than that of H–Br and H–I
bond.
The
bond dissociation of H – Cl bond is 103 Kcal/mole, which is higher than that of
HBr and HI.
So,
the H – Cl bond does not dissociate easily. Hence, HCl is thermally more stable
than HBr and HI.
Reducing properties of HCl acid
Since
the bond dissociation energy of H–Cl bond is higher than H–Br and H–I bond,
hence HCl acid is a mild reducing agents.
But
some oxidizing agent, such as KMnO4, K2Cr2O7 Pb3O4, PbO2etc, oxidize HCl acid
to release chlorine gas.
What is the cause of HCl acid strength ?
It
has been experimentally found that HCl acid is a strong acid. The HCl acid
solution change the litmus paper color from blue to red.In
aqueous solution, HCl acid is dissociated into the following ions, H3O+
and Cl–.
Now,
HCl acid is reacts with metallic carbonate or bicarbonate and evolved a effervescence
of colorless CO2 gas.
Besides,
HCl acid reacts with metal oxide and hydroxide to form salt and water.
The above reaction proved
that HCl is a strong acid which is also supported by the pKa
value of HCl acid [pKa = –7].
HCl acid preparation in laboratory
When a mixture of NaCl and concentrated H2SO4
is heated at different temperature, then hydrogen chloride is obtained.
This preparation method consisting of two step. In the first
step, NaCl react with H2SO4 at 150ᵒ-200ᵒC temperature,
produced NaHSO4 and HCl.
In the second step, the rest of the NaCl reacts with the
producing NaHSO4 under 500ᵒ-600ᵒC temperature and produce the
expected HCl gas.
When HCl gas is dissolved in water, then HCl acid is
prepared.
Industrial method for preparation of HCl acid
HCl acid is also prepared industrially by heating a mixture
of equimolecular H2 and Cl2 gas.
This is the large scale preparation method of HCl acid in
the world.
HCl acid application in chemistry
HCl acid is an important chemical in
chemistry. So, HCl acid has a significant role both in organic and in inorganicchemistry.
HCl acid is very important in industrial production of different goods. Some important application of HCl acid is
discussed below.
Important application as a laboratory reagent
HCl
acid is used as an important laboratory reagent. It is used as a reducing
agent, catalytic reagent, hydrolyzing agent, in the preparation and
identification of many organic compounds.
For
example, anhydrous ZnCl2 and concentrated HCl acid mixture[which is known as
Luis reagent],is used for identification of primary, secondary and tertiary
aliphatic alcohol.
Important application of HCl acid in industrial purpose
HCl
acid is used in industrial production of some important substance, such as, Cl2,
NH4Cl, glucose etc.
Again, in galvanization process, HCl acid is
used as a cleaning substance. It is used to clean iron sheet before tin plating
or galvanization.
Most
important use of HCl acid is in the dyeing industry, in the medicine production
industry and in sugar industry.
The
mixture of concentrated HCl acid and concentrated HNO3 in 3: 1 ratio[ known as
aqua regia ], is used as a melting agent of gold, silver etc in industry.
HCl is used in to prepare of chlorine gas
HCl acid is used to prepare Cl2
gas, oxidizing it by air oxygen in presence of CuCl2 catalyst in
Deacon’s process.
Cl2
gas is also produced on hydrolysis of concentrated HCl acid, in reaction with
some electro-positive metals [ Zn, Mg,
Ca etc ].
Others application of HCl acid.
Beside
the above uses, HCl acid is used in industry for removal of lubricants from animal
bone and also use for its purification.
HCl acid is used to prepare many organic compounds, such as alkyl chloride from alkene
and alcohol and glucose, fructose from sugar.
Important description of HCl acid in chemistry
Important
properties HCl acid in chemistry
Melting and boiling point of HCl
acid
Bond dissociation energy and
stability
Reducing
properties of HCl acid
What is the cause of HCl acid
strength?
HCl
acid preparation in laboratory
HCl
acid application in chemistry
Important application as a
laboratory reagent
Important application of HCl
acid in industrial purpose
HCl is used in to prepare of
chlorine gas













No comments