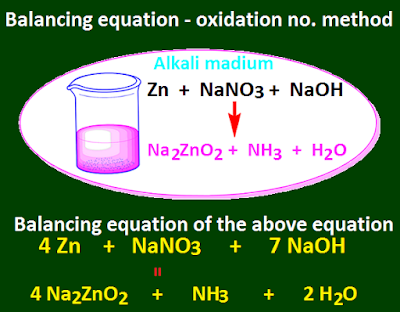
Balancing equations chemistry by oxidation number method for class 11
An
important method of balancing equations of chemical reactions is the oxidation
number system. In this method, the balancing of reactions is provided by
changing the oxidation number.
In the case of the oxidation number method, an
equation of the reaction is created by first identifying the reactants and the
products.
Then
the oxidation number of the atoms of the elements in the reactant and the
product changes, they are marked and written with oxidation numbers.
The
reactant in which the oxidation number of atoms of an element decreases is
oxidant and the reactant in which the atomic number of atoms in an element
increases acts as a reducing agent.
Oxidation
and reduction are complementary to each other. This is why the decrease
increase in oxidation number has to be equal.
For this reason, symbols of oxidant and reductant are multiplied by the suitable smallest integer, so that the change
in the oxidation number of both is equal.
Due
to balancing of equations often the symbols of other participating substances
in the reaction also need to be multiplied by the appropriate number.
In
the case of acidic medium reactions, to equalize the number of oxygen-atoms,
one H2O molecule is added for each oxygen-atom on the side of the
equation where there is an oxygen-atom deficit.