What is ethylene oxide or oxiran?
What is ethylene oxide or oxiran?
Ethylene oxide or oxiran is an organic compound with chemical formula C2H4O. It is a colorless and flammable gas with a faintly sweet ether-like odor.
Ethylene oxide or epoxy-ethane is a cyclic ether and the simplest epoxide that contains a three member ring consisting of one oxygen atom and two carbon atoms.
Ethylene oxide is isomeric with vinyl alcohol and also with acetaldehyde. It is industrially produced by oxidation of ethylene in presence of silver catalyst.
Ethylene oxide is liquid below 283 K temperature. The melting point and the boiling point of ethylene oxide are 160.7 K and 283.7 K respectively.
Read more : Methyl alcohol details in organic chemistry.
It is miscible in water. The density of ethylene oxide is o.88 g / cc. It is a polar compound. The dipole moment of ethylene oxide is 1.94 D.
Ethylene oxide is a very hazardous substance. It is a flammable, carcinogenic, mutagenic, irritating and anaesthetic gas.
Ethylene oxide structure with explanation
The molecular formula of ethylene oxide or oxiran is C2H4O. That is, the compound contains two carbon atoms, one oxygen atom and four hydrogen atoms. The molar mass of ethylene oxide is 44.052 g / mol.
In case of ethylene oxide, an oxygen atom is linked to two of the carbon atoms in a carbon chain resulting in the formation of a cyclic compound. Each carbon atom as well as oxygen atom of ethylene oxide are sp3 hybridized.
The H – C – H bond angle and C – O – C bond angle of ethylene oxide are 116.9ᵒ and 61.6ᵒ respectively. The C – C bond distance and C – O bond distance are 1.46Å and 1.43Å respectively.
The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 61° and a significant angular strain corresponding to the energy of 105 kJ / mol.
Ethylene oxide preparation
There
are some important methods for the preparation of ethylene oxide. In general,
it is prepared by epoxidation of ethylene (alkene) with peroxy acids, such as,
per-benzoic, p-nitro per-benzoic acid, peroxytrifluoroacetic acid etc.
Ethylene oxide may be prepared by the reaction of 2-chloroethanol and potassium hydroxide (KOH).
Ethylene
oxide is also manufactured by passing ethylene and air under pressure over a
silver catalyst at 200-400ᵒC.
Although ethylene oxide is dangerous for household use, yet it is used in the manufacture of many consumer products and non-consumer chemicals.
These products include detergents, condensers, solvents, plastics and various bio-chemicals such as ethylene glycol, ethanol-amines, simple and complex glycos, poly-glycol ethers and other compounds.
Ethylene oxide is a surface disinfectant that is widely used in the hospital and medical equipment industry for steam replacement in the disinfection of heat-sensitive equipment and tools.
It is so combustible and highly explosive that it is used as a major component of thermo-baric weapons.
Industrial uses
Ethylene oxide is one of the most important raw materials used in large-scale chemical production.
Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol. Other important products include ethylene glycol ethers, ethanolamines and ethoxylates.
Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate, liquid coolants and solvents.
Poly-ethylene-glycols are used in perfumes, cosmetics, pharmaceuticals, lubricants, paint thinners and plasticizers.
Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers and paints.
Other products of oxiran (ethanolamines) are used in the manufacture of soap and detergents and for purification of natural gas.
Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids or amines. They are used in the manufacture of detergents, surfactants, emulsifiers and dispersants.
Non-industrial uses
Ethylene oxide is used as a sterilizing agent, disinfecting agent and fumigant as a mixture with carbon dioxide, nitrogen or dichlorodifluoromethane.
It is applied for gas-phase sterilization of medical equipment and instruments, packaging materials and clothing, surgical and scientific equipment for processing of storage facilities, clothing, furs and valuable documents.
Healthcare sterilant
Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry due to its non-damaging effects for delicate instruments and devices that require sterilization.
It is used for instruments that cannot tolerate heat, moisture or abrasive chemicals, such as electronics, optical equipment, paper, rubber and plastics.
In the United States, the operation of ethylene oxide sterilization is overseen by the EPA through the National Emission Standard for Hazardous Air Pollutants.
Ethylene oxide is used as a fungicide and as an accelerator of maturation of tobacco leaves. Ethylene oxide is also used as a main component of thermo-baric weapons.
Other uses
Ethylene oxide is an important reagent in organic chemistry. It is used in the preparation of primary alcohol, amine and aldehyde with higher molecular weight.
It is also used to prepare 2-methoxy ethanol, 2-ammino ethanol, 2-bromo ethanol, ethane-1,2-diol, 3-hydroxy propane nitrile etc.
Ethylene oxide reactions
Ethylene oxide is a very important organic reagent. It undergoes a large numbers of chemical reactions.
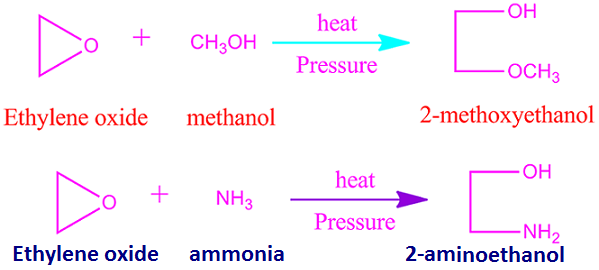
When
ethylene oxide is heated with methanol and ammonia individually under pressure,
then 2-methoxy ethanol and 2-ammino ethanol are obtained.
Again,
on hydrolysis, ethylene oxide gives ethane-1,2-diol. When it is heated with
aqueous HBr, 2-bromo ethanol is the product.
The most important reaction of ethylene oxide is the reaction with Grignard reagent through which the enhancement of carbon atom takes place in an organic compound.
These
reactions are applied for the preparation of long chain primary alcohol,
primary ammine and aldehyde compounds.
What is ethylene oxide sterilization?
Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry due to its non-damaging effects for delicate instruments and devices that require sterilization.
It is used for instruments that cannot tolerate heat, moisture or abrasive chemicals, such as electronics, optical equipment, paper, rubber and plastics.
Ethylene oxide sterilization is also called EtO. EtO gas is carcinogenic, explosive and mutagenic. The use of poisonous gasses should remain limited to sterilizing products for which no alternative methods are available.
The obvious reason is that whatever kills microorganisms is lethal to humans as well.
Most sterilization chemicals and gases are already endangering human health and even lives in very low concentrations.
In spite of this, ethylene oxide sterilization method is used. Advancement in medical procedures has resulted in the increased usage of delicate instruments which cannot be steam sterilized.
This is because they cannot withstand the higher temperatures or moisture of steam. Heat and moisture sensitive equipment require alternative methods of sterilization.
Ethylene Oxide (EtO) is a common gas used for low temperature sterilization. It is a colorless, poisonous gas that attacks the cellular proteins and nucleic acids of microorganisms.
It is most commonly used to sterilize instruments with long lumens such as endoscopes and all materials that have to be sterilized but cannot withstand higher temperature.
EtO process temperatures from 30 - 50°C are used. A lower temperature results in a less efficient process which leads to a longer exposure time.
EtO
sterilization cycle consists of three typical stages, preconditioning,
sterilization, aeration. The cycle time is usually more than 14 hours.
- What is ethylene oxide or oxiran?
- Ethylene oxide structure with explanation
- Ethylene oxide preparation
- Ethylene oxide uses
- Ethylene oxide reactions
- What is ethylene oxide sterilization?
ethylene
oxide or oxiran, epoxy-ethane, ethylene oxide structure with explanation, ethylene
oxide preparation, ethylene oxide uses, ethylene oxide reactions, ethylene
oxide sterilization,














Get the BEST JOINT HIP REPLACEMENT SURGERY ,knee replacement surgery and other orthopedic surgeries by top orthopedic surgeons in Bhargava medical & trauma Centre, Kanpur having more than 15 years of experience in this field. best orthopedic hospital in kanpur.
ReplyDeleteHi,
ReplyDeleteI read your article and its so well written. I have also written something like yours. It would really be helpful if you could read my blog on Ear Surgery in kanpur and give your suggestions.
Thank You!