Sodium carbonate preparation in Solvay process
Sodium carbonate preparation in Solvay process
Sodium
carbonate is an important inorganic compound with chemical
formula, Na2CO3.
Earlier it was called soapstone (sajimati). It is also called
washing soda, soda ash, soda crystals etc. Sodium
carbonate is a very important laboratory reagent.
It
also has many important uses. This is why Na2CO3 is
prepared in large scale industrial processes. Industrial production of Na2CO3 is done by
three methods. For example, Solvay
method, electrolytic method and Le Blanc method.
Among these methods, Na2CO3
preparation by Solvay method is the
simplest and least expensive. Solvay process is also called ammonia soda
process.
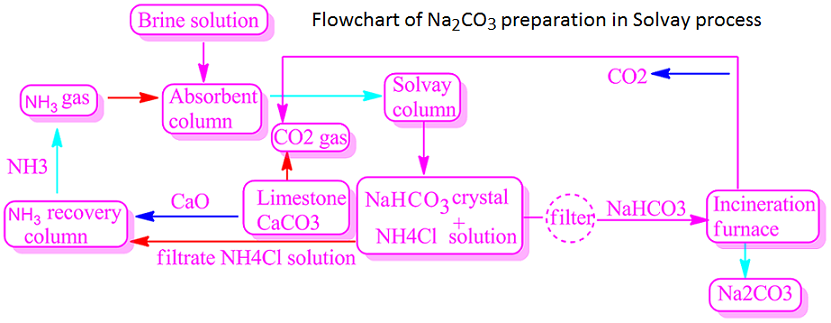
Solvay Process :
The entire process is consists of five steps. In the first step, an aqueous solution of NaCl or brine solution is prepared. In the second step, the solution is saturated by passing NH3 gas into the saturated brine solution.
In the third step, CO2 gas is
passed through the ammonia saturated brine solution. As a result NH4HCO3 is
obtained. The reaction takes place under 30-35°C temperature.
The producing ammonium bicarbonate is reacts with NaCl resulting in the formation of NaHCO3 precipitate and NH4Cl. Both are reversible reactions.
NH3 + CO2 + H2O ⟶ NH4HCO3
NH4HCO3 + NaCl ⟶ NaHCO3 + NH4Cl
The NaHCO3 precipitate thus obtained is filtered and it washed well with water. It is then dried thoroughly. In the fourth step, the dry NaHCO3 precipitate is heated to 180°C to dissociate to produce Na2CO3.
2NaHCO3 ⟶ Na2CO3 + CO2 ↑ + H2O
In the last step, NH3 is produced from NH4Cl present in the filtrate solution. In this case, CaO, a stronger alkali than NH3, is used. Again, the by-product CaO is used to produce CO2 from limestone.
2NH4Cl + CaO = 2NH3 + CaCl2 + H2O
CaCO3 → CaO + CO2 ↑
Ammonia produced in the Solvay process is recycled.
This process only takes brine and
limestone. Calcium chloride is its only waste product. The process is
substantially more economical than the LeBlanc process.
The LeBlanc process produces two waste products, calcium sulfide and hydrogen chloride. The Solvay process dominates the production of large quantities of sodium carbonate worldwide.
Sodium carbonate, Na2CO3
preparation, solvay process
- What is sodium carbonate?
- How Na2CO3 is prepared in Solvay process?








No comments