What is kinetically and thermodynamically controlled reaction ?
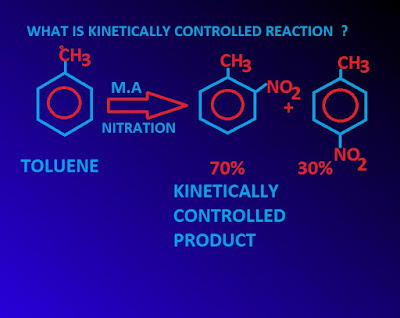
What is kinetically controlled reaction ?
When a chemical reaction gives rise to alternative products, the proportion of different products of that reaction are very often determined by their relatives rates of formation.
Usually the faster a product is formed, the greater is its proportion in the product mixture.
When the mixture of the product or the proportion of the products in the final product mixture are determined from the rate of formation, it is said to be kinetically controlled product and the concerned reaction is called kinetically controlled reaction.
For example, the addition of
HBr to C = C bond in prop-1-ene
in the presence of organic peroxide that is , the radical addition of HBr to
prop-1-ene may produce 1-bromopropane or
2-bromopropane are as follows,
In this reaction hydrogen radical (Hᵒ
) and bromine radical ( Brᵒ ) are added to C = C
bond by radical mechanism.
Since the bromine radical( Brᵒ ) moves faster than the hydrogen radical (Hᵒ ) and CH3CᵒHCH2Br radical is more stable than the CH3CHBrCᵒH2 radical, CH3CᵒHCH2Br radical forms faster than CH3CHBrCᵒH2 radical.
Since the bromine radical( Brᵒ ) moves faster than the hydrogen radical (Hᵒ ) and CH3CᵒHCH2Br radical is more stable than the CH3CHBrCᵒH2 radical, CH3CᵒHCH2Br radical forms faster than CH3CHBrCᵒH2 radical.
Consequently, Hᵒ radical attacks CH3CᵒHCH2Br readily and 1-bromopropane is the product that is kinetically controlled
product.
What is thermodynamically controlled reaction?
When a chemical reaction gives rise to alternative products,
the nature of the products and their proportion depends on the energetic of the reaction that is on the kinetic andthermodynamics of the reaction.
If the reaction is reversible or if the products are readily inter convertible , under the same condition of the reaction, the thermodynamicstabilities of the products determine the nature and the proportion of the products .
The greater is the thermodynamic stability of a product, the greater is its proportion in the final product mixture.
Thus, if the amount of product is determined by their relative thermodynamic stabilities, it is known as thermodynamically controlled product and the concerned reaction is said to be thermodynamically controlled reaction.
For example, Friedel-crafts alkylation of toluene is thermodynamically controlled reaction.
When toluene is treated with
methyl chloride in the presence
of anhydrous AlCl3 as
catalyst, after 10 second the products mixture is found to contain o-xylene 23%
, m-xylene 46% and p-xylene 31% , while
after one second the percentage of o, m and p-xylenes are 40%, 21% and 39%
respectively.
Since thermodynamically m-xylene is the more stable compound among the three, the proportion of the products after 10 second is definitely due to relative thermodynamic stability though the rates of formation of ‘o’ and ‘p’ isomers are almost equal and that of ‘m’ isomer lags behind.
Summary
What is kinetically controlled reaction ?









No comments