Calculate the spin only magnetic momentum µ of K3 [ Mn(CN)6] compound .
Calculate
the spin only magnetic momentum µ of K3[ Mn(CN)6] compound .
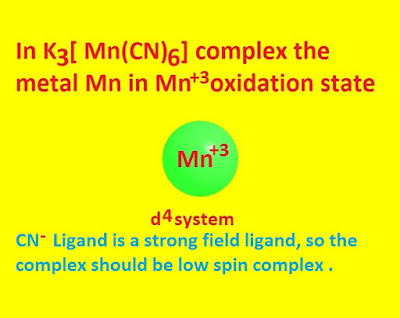
In K3 [ Mn(CN)6] the metal
manganese in Mn +3
oxidation state . Mn +3 ion is a d 4 system . Cyanide ion
is a very strong field ligand .
According to crystal field theory the given
complex will be of low spin type and its
electron distribution will be t2g4 eg0
which is shown below.
There is two unpaired electron in the
complex , it will be paramagnetic and magnetic moment will correspond to two
unpaired electron which is given by µ .
Why tetrahedral metal complexes are usually not of low spin ?
According to crystal field theory splitting of d-orbitals takes place during complexation and they split into two
different sets of orbitals in both octahedral and tetrahedral complexes.
The
value of splitting energy depends on the nature of the metal ion ligand and the
nature of the geometry of the complexes.
Depending of the value of this
splitting energy Δo ( for octahedral ) and Δt ( for tetrahedral ) high spin and
low spin complexes may result.
As for example , for an octahedral complex of d4 system the electron distribution may either be t2g3 eg1 ( high spin) when Δo < p or t2g4 eg0 (low spin ) when Δo > p .
As for example , for an octahedral complex of d4 system the electron distribution may either be t2g3 eg1 ( high spin) when Δo < p or t2g4 eg0 (low spin ) when Δo > p .
Spin pairing energies of different d4-system of a given d-series of
transition element are almost the same.
Therefore it is necessary to obtain
high spin low spin complexes the value of
Δo should be greater than pairing energy, p ( low spin ) in some cases
and also it should be less than ,p ( high spin ) in some cases. Actually these
are possible in octahedral complexes.
It has been found that Δt is always
very small and is about 45% of Δo in all cases and never exceeds pairing
energy.
Therefore these is no possibility of
formation of low spin complexes . So it can be said that all tetrahedral metal
complexes are usually not of low spin.
Summary.
Calculate
the spin only magnetic momentum µ of K3
[ Mn(CN)6] compound .









This is a wonderful and amazing blog post site. thanks for sharing this blog post site.
ReplyDeletehttps://www.lovingparents.in/environmental/positive-impact-of-coronavirus-on-environment/
94E39D9DA9
ReplyDeleteTakipçi Satın Al
Sevgiliyle Oynanacak Oyunlar
Promosyon Kodu
En İyi Yabancı Diziler
Cami Şamdanı
En İyi Ücretsiz Oyunlar
İçki Fiyatları
Offshore Hosting
Takipçi Satın Al