What is sodide ion ? How sodide ion stabilized in solution ?
What is sodide ion ? How sodide ion stabilized in solution ?
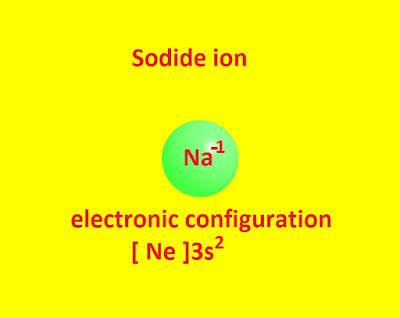
The sodide ion is Na -1. It is an anion like non metallic anions such as Cl - , Br -, I – etc .
It has the electronic configuration [ Ne ] 3s 2. Group (I) metals ( Na , K , Rb ) are stable in liquid NH3.
The solution is blue when dilute. The solution conduct electricity and are paramagnetic.
These measurement of transport number suggest that the main carrier is solvated electrons by which is implied electrons which are free from their parents Na-atoms occupy cavities in the ligand.
Electron spin resonance
studies have shown the presence of free electrons , but the decrease of para
magnetism with increasing
concentration suggest that ammoniated electrons can associated to far diamagnetic electron pairs.
concentration suggest that ammoniated electrons can associated to far diamagnetic electron pairs.
How the electrons are associated with the ammonia molecule ?
A suggestion has been
made that in addition to solvated cations negative ions stabilized by solution
may exist in dilute solution .
This can be involved in an oxidation-reduction equilibrium involving the solvent S’ with S -1 ( solvated electron )
This can be involved in an oxidation-reduction equilibrium involving the solvent S’ with S -1 ( solvated electron )
⇆
M + + 2 S - M - + 2 S
M + + 2 S - M - + 2 S
In addition ion
pairing equilibrium are involved .
⇋ M + + S - M + S-
M + + M- ⇋ M+ M-
Where M= Na , LI, K ….. etc.
Fairly stable other such solution of group I ( A ) metals Containing M – ions can be obtained with solvents like tetrahydrofuran ( THF) ethylene diethyl ether and orther methyl polyethers.
Summary
What is Sodide ion ?
How sodide ion stabilized in solution ?








No comments