How do you explained the bonding of the carbonyl groups in the structure of Fe2(CO)9 through IR-spectra ?
How do you explained the bonding of the carbonyl groups in the structure of Fe2(CO)9 through IR-spectra ?
The sixth member of 3d transition series is iron (Fe). Few properties of elemental iron are shown below pictorially,
Iron formed few carbonyl compounds like Fe(CO)5
, Fe2(CO)9 etc. The most important carbonyl compound of iron is diiron nona carbonyl ,Fe2(CO)9 .
Infrared
and x-ray studies have shown that in
this organometallic compound each Fe
atom is directly linked with the other Fe atom by a sigma bond ( bond distance 2.46 Å ) to three bridging carbonyl ( CO ) groups by a sigma bond and for three terminal
carbonyl groups by a coordinated bond.
Presence of both type of carbonyl group ,that is,bridging and terminal can be easily proved with the help of I.R-spectra of the molecule.
Presence of both type of carbonyl group ,that is,bridging and terminal can be easily proved with the help of I.R-spectra of the molecule.
In bridging or ketonic carbonyl groups , there is no possibility or
necessity of back bonding , C – O bond
order is purely 2. The stretching frequency of such group is in the order of 1700 – 1850
cm -1 .
In terminal carbonyl groups , there is a back bonding due to which the bond order of terminal carbonyl group is reduced to some extent from the value of three , which is the bond order of free CO molecule.
In terminal carbonyl groups , there is a back bonding due to which the bond order of terminal carbonyl group is reduced to some extent from the value of three , which is the bond order of free CO molecule.
Due to
this back bonding stretching frequency of free CO molecule ( 2143 cm -1
) is reduced, but obviously remains
above 1850 cm -1.
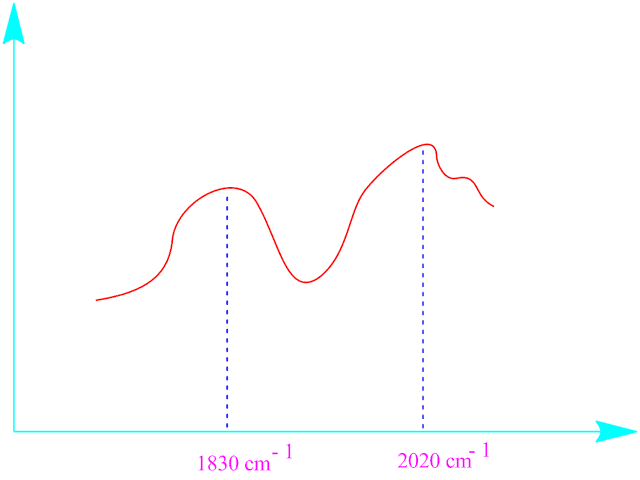
The presence of two absorbance peaks one at around 1830 cm -1 for bridging CO group and other at 2020 cm -1 for terminal CO group .
This two value confirms the above structure of di-iron nona carbonyl ,Fe2(CO)9 .
The presence of two absorbance peaks one at around 1830 cm -1 for bridging CO group and other at 2020 cm -1 for terminal CO group .
This two value confirms the above structure of di-iron nona carbonyl ,Fe2(CO)9 .
The
absorbance curve of stretching frequency of bridging and terminal carbonyl
groups in the structure of Fe2(CO)9 are given
below.
Practice problem:
Discuss the structure and bonding of Fe2(CO)9 .
Write down the structure of iron pentacarbonyl .









No comments