Why most of the transition metals are used as catalysts ?
Why most of the transition metals are used as catalysts ?
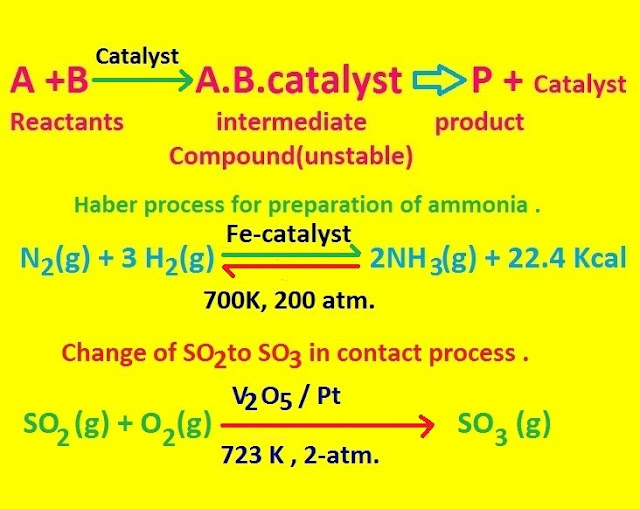
According to
the modern theory of catalysis , a catalytic substance is capable of
forming an unstable intermediate compound which readily decomposes yielding the product and regenerating the catalyst.
forming an unstable intermediate compound which readily decomposes yielding the product and regenerating the catalyst.
The
transition elements, on account of their variable valency , are able to form
unstable intermediate compounds very readily.
These
elements can also provide a large surface area for the reactants to be adsorbed
and thus come closer to one another for the reaction to occur readily on the surface of the catalyst itself.
and thus come closer to one another for the reaction to occur readily on the surface of the catalyst itself.
For the
above reason, most of the transition metals and their compounds have good
catalytic properties and used as catalyst. Platinum, iron, vanadium pentoxide nickel etc, are important examples.
catalytic properties and used as catalyst. Platinum, iron, vanadium pentoxide nickel etc, are important examples.
Platinum is
a general catalyst and is used particularly in the contact process involving combination
of sulphur dioxide and oxygen to yield sulphur trioxide .
Vanadiumpentoxide [ V2O5 ] is also a good catalyst for the same reaction.
Iron catalyses the combination of nitrogen and hydrogen in the Haber process for the production of ammonia. Nickel is a good catalyst in hydrogenation.
Iron catalyses the combination of nitrogen and hydrogen in the Haber process for the production of ammonia. Nickel is a good catalyst in hydrogenation.
Why ZnCl2 is fully ionized in dilute aqueous acidic solution but HgCl2 is not ?
Zinc (Zn ) and mercury ( Hg ) are the members of same family of group IIB . ZnCl2 is fully ionized in dilute aqueous acidic solution because it behave as electrolytes in such solution.
But HgCl2 remains unionized in aqueous solution . Since the solution does not conduct electricity .
Due to lanthanide contraction and ineffective shielding of 4f14 and 5d 10 electrons,
ionization potential and electronegativity of Hg becomes higher than that of Zn , which is reverse to the group trend .
Mercury is
thus less metallic or more non metallic than Zinc . ZnCl 2 has a
considerable ionic character but Hg – Cl bonding is mostly covalent .
This
strong covalent nature of HgCl2 prevent its ionization in aqueous solution.
Thus aqueous
solution of HgCl2 is a non
electrolyte like sugar solution .
Summary :
Why most of the transition metals are used as catalysts ?
Why ZnCl2
is fully ionized in dilute aqueous acidic solution but HgCl2 is not ?







No comments