Calculate the number of unpaired electrons and LFSE of [ Fe ( H2O)6 ]+3 ion .
Calculate the number of unpaired electrons and LFSE of [ Fe ( H2O)6 ]+3 ion .
The
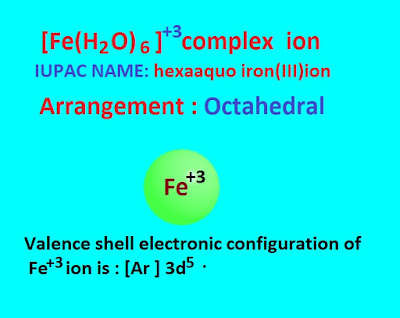
IUPAC name of the above complex ion is
hexaaquo iron(III) ion. The oxidation state of iron in this complex ion is +3 .
The valence
shell electronic configuration of Fe +3
ion : [Ar] 3d5 .
The arrangement of the complex ion is octahedral . The octahedral structure of this complex are as follows ,
The arrangement of the complex ion is octahedral . The octahedral structure of this complex are as follows ,
Ligand H2O,
which is a weak field Ligand . Under the
influence of the octahedral crystal field, the five degenerate d-orbital’s of Fe +3 ion are splitted into two sets of energetically
different orbital’s.
These two sets are energetically lower t2g orbital and energetically higher eg orbital .
These two sets are energetically lower t2g orbital and energetically higher eg orbital .
As there are
five electrons in the 3d5 degenerate orbital’s ,
so according to crystal field theory the possible arrangement is t2g3 eg2
.
Here, the
crystal field splitting energy ( 10 Dqo ) is lower than pairing
energy (P).
That is , 10
Dqo < P
So, no
pairing of electrons will be occured in
any sets of orbital’s .
The crystal
field splitting of degenerate d-orbital’s of
Fe +3 ion under the
influence of Ligand weak field Ligand H2O
are as follows,
Since H2O is a
weak field Ligand , so it should be high spin complex
and from the above splitting diagram ,it is clear that the complex ion
have five unpaired electron .
Due to presence of five unpaired electron , the [ Fe( H2O)6
]+3 complex ion exhibit paramagnetic properties .
Calculation of Ligand field stabilization energy .
The Ligand field
stabilization energy ( LFSE ) of the above complex ion is,
3x( -4 Dqo ) +
2 x 6 Dqo
= - 12 Dqo + 12 Dqo
= 0 Dqo .
Summary : = - 12 Dqo + 12 Dqo
= 0 Dqo .
Calculate
the number of unpaired electrons and LFSE of [ Fe ( H2O)6
]+3 ion .
What is ligand field stabilization energy ?Calculate the spin only magnetic momentum µ of K3 [ Mn(CN)6] compound .









No comments