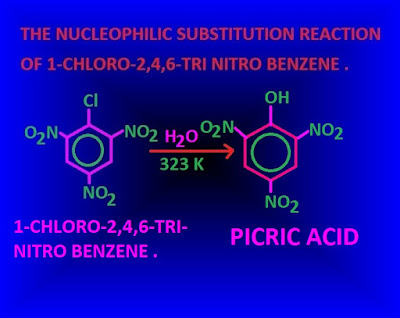
Why the nucleophilic substitution reaction of 1-chloro-2,4,6-trinitro benzene occurs very easily ?
Why the nucleophilic substitution reaction of 1-chloro-2,4,6-trinitro benzene occurs very easily?
1-chloro-2,4,6-trinitro benzene contains three nitro groups which are strong electron with drawing group.
Due to –R effect of this three nitro group , the density of electron of benzene nucleus very much decrease .
Therefore, the benzene nucleus becomes very
much active for nucleophilic substitution reaction.
Hence, a very weak nucleophile even water molecule as a nucleophile can attack the targeted carbon atom .
Hence, a very weak nucleophile even water molecule as a nucleophile can attack the targeted carbon atom .
Again, the carbanions produced by the attack of nucleophile ,gets stability through the resonance of three –NO2 group.
Consequently,
nucleophilic substitution reaction of 1-chloro-2,4,6-trinitro benzene occurs
very easily .
Why the basic hydrolysis of p-nitro chloro benzene occurs easily than chloro benzene ?
The hydrolysis of p-nitro chloro benzene and chloro benzene is an example of nucleophilic substitution reaction .
The nucleophilic substitution reaction is facilitated ,if the compound contain electron withdrawing group.
Now, in p-nitro chloro benzene, the –NO2 group is an electron withdrawing group.
Due to – R effect of –NO2 group ,the density of electron of
benzene nucleus decreases.
Therefore , the benzene ring becomes more active . As a result , the nucleophile can attack the targeted carbon atom easily.
Therefore , the benzene ring becomes more active . As a result , the nucleophile can attack the targeted carbon atom easily.
Reaction mechanism. Step:I
Step: II
Besides
this, the inter mediate carbanions produced by the attack of nucleophile ,
gains extra stability through resonance with the –NO2 group .
Consequently, the basic hydrolysis of p-nitro chloro benzene occurs easily than chloro benzene .
Why the melting point of p-dichlorobenzene is higher than ortho and meta isomers ?
The melting point of p-dichlorobenzene is higher than ortho and meta isomers because p-dichloro benzene is more symmetrical than ortho and meta isomers.
So p-dichloro benzene molecules are staying very much close to each other in the crystal lattice .
Therefore, the inter molecular force of attraction acts among the molecules is higher than that of ortho and meta isomers.
Hence, the amount
of energy required to break this crystal lattice of p-dichlorobenzene is higher
than o-dichloro benzene and m-dichloro benzene.
Consequently, the melting point of p-dichlorobenzene is higher than ortho and meta isomers.
Summary
- Why the nucleophilic substitution reaction of 1-chloro-2,4,6-tri nitro benzene occurs very easily ?
- Why the basic hydrolysis of p-nitro chloro benzene occurs easily than chloro benzene ?
- Why the m.p of p-dichloro benzene is higher than ortho and meta isomers ?












No comments