Why is chlorine, ortho and para directing and Why ‘Cl’-atom can not form H-bond but ‘N’-atom can ?
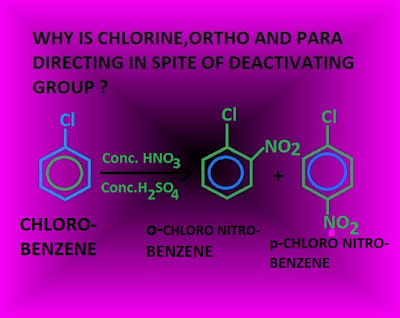
Why is chlorine, ortho and para directing in spite of deactivating group ?
Most of the reaction of chloro benzene [such as nitration, sulphonation, halogenations and Friedel craft reaction etc ] are electrophilic reaction. Now, –Cl group is a deactivating group.
Due
to – I effect, chlorine atom attract electron from benzene nucleus towards itself
.
But, due to
+ R effect ,the lone pair of ‘Cl’-atom participate in resonance with the π electron of benzene nucleus.
As a result ,the density of
electron increases on ortho and para carbon atom of benzene ring .
Consequently,
the electrophile attack on the ortho and para carbon atom of benzene nucleus .
Therefore ,
in spite of deactivating group chlorine atom acts as a ortho and para directing.
Although ,the para product is greater than ortho product due to more steric hindrance in ortho position .
For example, nitration of chloro benzene forms ortho chloro nitro benzene and para chloro
nitro benzene .
Aromatic nitration reaction is an electrophilic substitution reaction . Mechanism of aromatic nitration reaction for preparation of ortho product is shown below,
Which one is more active toward basic hydrolysis between chloro benzene and 1-chloro-2,4-dinitro chloro benzene ?
The hydrolysis of chloro benzene and 1-chloro-2,4-dinitro chloro benzene are example of aromatic nucleophilic substitution reaction . Now, –NO2 group strong electron withdrawing group .
So, due to – R effect of two nitro group , the density
of electron of benzene nucleus decreases .
As a result,
the benzene nucleus becomes more active and easily attacked by the nucleophile
.
Besides this,
the inter mediate carbo anions is produced by the attack of nucleophile , gains
extra stability through the resonance of two –NO2 groups.
But, in
case of chloro benzene, no such type of electron withdrawing groups are
present . Hence, the carbo anions produced is less stable with respect to carbanions produced from 1-chloro-2,4-dinitro chloro benzene.
Consequently, 1-chloro-2,4-dinitro chloro benzene is more effective towards basic
hydrolysis than chloro benzene.
What is the condition for the formation of hydrogen bond ?
There are
two main condition for the formation of hydrogen bond of any molecule.
(I ) The
molecule which participate in the formation of hydrogen bond must be contain a
specific hydrogen atom that is attached with a strong electronegative atom (
such as F, N , O etc ) and ,
(II)The atom
of the electronegative molecule should be small in size.
Why ‘Cl’-atom can not form H-bond but ‘N’-atom can ?
Fluorine,oxygen and nitrogen atoms are effective for the formation of hydrogen bond .
Because, only these three atoms can fulfill the sufficient condition for the
formation of hydrogen bond .
Now, both
chlorine and nitrogen atom are equally electronegative. That is their
electronegativity value in pauling scale is 3.
This is because of that ,the atomic radius of chlorine ( 0.99 Aᵒ ) is larger than the atomic radius of nitrogen ( 0.70 Aᵒ ), which does not permit the chlorine atom for the formation of stable hydrogen bond.
Summary
- Why is chlorine, ortho and para directing in spite of deactivating group ?
- Which one is more active toward basic hydrolysis between chloro benzene and 1-chloro-2,4-dinitro chloro benzene ?
- What is the condition for the formation of hydrogen bond ?
- Why ‘Cl’-atom can not form H-bond but ‘N’-atom can ?












No comments