Why C – O bond length in phenol is less than methanol and what is Bouveault Blanc reduction ?
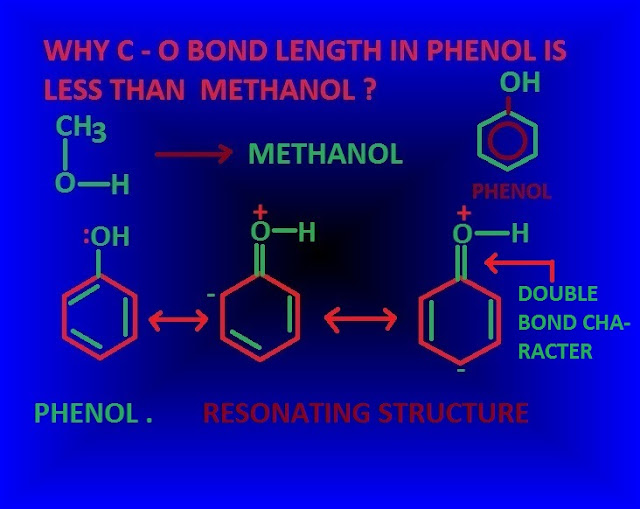
Why C – O bond length in phenol is less than methanol ?
In case of phenol, the lone pair on oxygen atom takes part in resonance with the π – electron of benzene ring . As a result, the C – O bond gets double bond character . So the C – O bond length decreases.
On the other
hand , no such type resonance is happened in methanol.
Consequently,
phenolic C – O bond ( 136 pm ) is slightly less than methanolic C – O ( 142 pm ) bond.
Why C – O – H bond angle of aliphatic alcohol is less than pure tetrahedral bond angle ?
In alcohol
molecule , both the ‘C’ and ‘O’-atom are sp3 hybridized . Among the four sp3
orbital of ‘O’-atom, two orbital overlap with 1s orbital of hydrogen and sp3
orbital of ‘C’ atom respectively.
As a result, O – H and O – C sigma bond is formed . The remaining two sp3 orbital contain one lone pair of electron .
According to
VSEPR theory, lone pair – lone pair repulsion is greater than lone pair – bond
pair repulsion . Hence the C – O – H bond angle is slightly decreases from the
pure tetrahedral bond angle ( 109ᵒ 28’ ).
What is Bouveault Blanc reduction ?
On reduction
of ester compound gives two moles of same or different alcohol molecules . This
reaction is known as Bouveault Blanc reduction .
In this
reaction , the reducing agent is used Na / C2H5OH or
LiAlH4 copper chromite ( CuO.CuCr2O4 ) at high
pressure.
In Bouveault Blanc reduction, the acyl part of
the concerned ester is always convert into primary alcohol and the alkoxy part
(- OR’) of the ester reduced into
primary, secondary or tertiary alcohol.
The reduction of aldehyde or ketone by Na / C2H5OH is also known as Bouveault Blanc reduction.
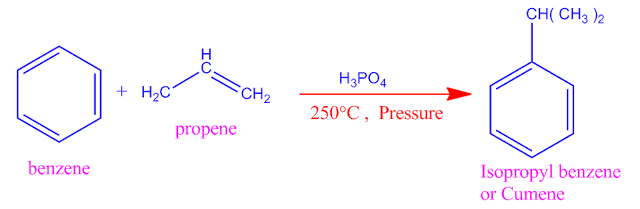
What is cumene phenol process ?
When benzene
under goes Friedel craft alkylation in the presence of phosphoric acid and
propene , isopropyl benzene or cumene is obtained.
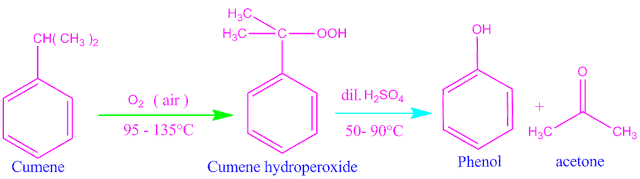
If we passed air through cumene solution , it oxidized by oxygen convert into cumene hydro peroxide.
On addition of 10% sulfuric acid, cumene hydro peroxide under goes hydrolytic rearrangement and finally produced phenol as a main product along with acetone as a side product.
Application of cumene phenol process
This is an very important process for the preparation of phenol. In this process, two very essential or important organic compound such as phenol and acetone are prepared from two available organic compounds, benzene and propene respectively.
The most
amount of phenol in the world is produced by this method.
- Why C – O bond length in phenol is less than aliphatic alcohol ?
- Why C – O bond length in phenol is less than methanol ?
- Why C – O – H bond angle of aliphatic alcohol is less than pure tetrahedral bond angle ?
- What is Bouveault Blanc reduction ?
- What is cumene phenol process ?



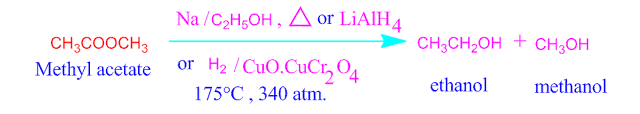






Hi,
ReplyDeleteI read your article and its so well written. I have also written something like yours. It would really be helpful if you could read my blog on Ear Surgery in kanpur and give your suggestions.
Thank You!