Covalent bond-polar covalent bond-non polar covalent bond-examples
·
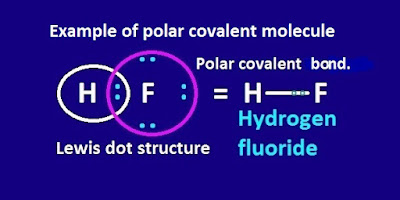
What is polar covalent bond ?
If
a covalent bond is formed by sharing of electron between two dissimilar or
different atoms, then this covalent bond is called polar covalent bond.
Now,
more electronegative atom have a tendency to attract the bonded electron pair
towards itself.
As
a result, the shared electron partially moved towards the more electronegative
atom.
Due
to this unsymmetrical charge distribution, more electronegative element gets
partial negative charge and the less electronegative element gets partial
positive charge.
In
this way, the covalent bond change into polar covalent bond and the molecule
becomes polar molecule.
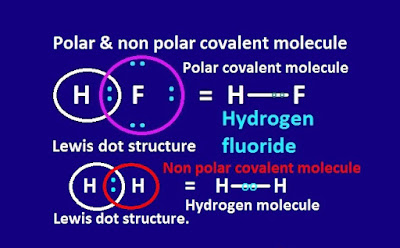
For example, Hᵟ+ – Fᵟ- , Hᵟ+ – Clᵟ- , Hᵟ+– Brᵟ- etc molecules contain polar covalent bond.
The
polar covalent bond is, therefore, has partial ionic character .
The two opposites charges at the ends are called electrical poles and the molecules are called dipolar molecules.
The two opposites charges at the ends are called electrical poles and the molecules are called dipolar molecules.
· What is non polar covalent bond ?
Covalent
bond is formed by sharing of electron between two similar or dissimilar atoms.
There are two types of covalent bond, namely, non polar covalent bond and polar
covalent bond.
The
covalent bond is formed by sharing of electron between two identical or similar
atoms, is said to be non polar covalent bond.
In
this type of covalent bonding, the shared electron pair is attracted equally by
both the atoms and lies exactly midway between them.
The
resulting covalent compound is called non polar molecule.
For
example, hydrogen , chlorine , bromine, oxygen, nitrogen etc molecule contain
non polar covalent bond.
·
What is polar and non polar molecules ?
If
a covalent bond is formed by sharing of electron between two identical or
similar atoms, then the covalent bond is said to be non polar covalent bond and the resulting covalent compound is called non polar molecule.
similar atoms, then the covalent bond is said to be non polar covalent bond and the resulting covalent compound is called non polar molecule.
For
example, hydrogen , chlorine , bromine, oxygen, nitrogen etc molecule contain
non polar covalent bond.
On
the other hand, if a covalent bond is formed by sharing of electron between
two dissimilar or different atoms, then this covalent bond is called polar covalent bond and the resulting covalent compound is called polar molecule.
two dissimilar or different atoms, then this covalent bond is called polar covalent bond and the resulting covalent compound is called polar molecule.
In
this type of molecule, the polarity arises due to difference in electro-negativity of the two dissimilar atoms.
For
example, Hᵟ+ – Fᵟ- , Hᵟ+ – Clᵟ- , Hᵟ+– Brᵟ- etc
molecules are polar covalent molecules.
Summary :
- · What is polar covalent bond ?
- · What is non polar covalent bond ?
- · What is polar and non polar molecules ?








No comments