TlCl more stable thanTlCl3
Why is TlCl more stable than TlCl3 molecule?
‘Tl’ is a group 13th or p-block heavier element. The outer electronic configuration of thallium is, [Xe] 4f14 5d10 6s26p1.
From
the electronic arrangement of Tl, it has been found that, the valence
electron of thallium element is 3.
So,
the oxidation state of Tl should be +3 . But it has been found that , +3 oxidation
state of Tl is less stable than
+1 state.
Because,
‘Tl’ contained inner ‘d’ or ‘f’ electrons.
Now,
due to poor shielding effect of ‘d’ or ‘f’ orbitals, the effective nuclear charge on outer most ‘s’-electrons act strongly.
That is, the nucleus bind or pull the ‘s’-electrons
strongly. Hence , this two ‘s’-electron
remain inert and does not participate in chemical reaction.
Since
+3 oxidation state of Tl is less
stable than +1 oxidation state, so TlCl is more stable than TlCl3 molecule.
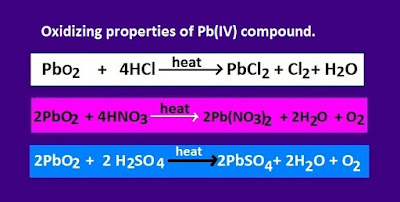
Why is PbO2 act as a strong oxidizing substance ?
The outer electronic configuration of lead is, [Xe] 4f14 5d106s26p2.
From
the electronic arrangement of lead, it has been found that, the valence
electron of lead is 4. So, the
oxidation number of lead should be +4 .
But
it has been found that, the +4 oxidation
state of lead is unstable than +2, due to inert pair effect.
PbO2
is so strong oxidizing that, it can oxidized even concentrated sulfuric acid ,nitric acid and hydrochloric acid.
Through
these oxidation, lead changes its
unstable oxidation state[+4] to stable oxidation state[+2 ].
Why Po(VI) compound act as a strong oxidizing substance ?
The outer
electronic configuration of polonium
is, [Xe] 4f14 5d10
6s26p4.
So, the
maximum oxidation state of polonium may
be +6. But, it is very unstable oxidation state.
Because, the
outer ‘6s’-electron of polonium have
no tendency to participate in chemical reaction due to inert pair effect.
Hence, Po(VI) compound act as a strong oxidizing agent in inorganic
chemistry.
That is, Po(VI) compound have great tendency
to reduced the other substance, so that, it can changes its unstable +VI oxidation state to stable +IV oxidation state.
For this
purpose, Po(VI) compound [ PoF6
] act as a strong oxidizing
substance.
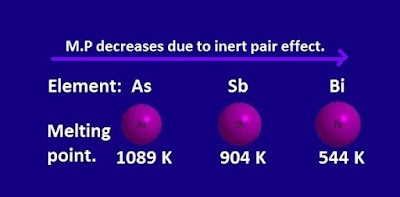
Why is the melting point of ‘Sb’ and ‘Bi’ elements less than ‘As’ element ?
The
melting point of ‘Sb’ and ‘Bi’
elements are less than ‘As’ element.Because of the inertpair effect, the ‘Sb’ and ‘Bi’ elements form three covalent bond instead of
five.
So, in ‘Sb’ and ‘Bi’ elements, the
inter-atomic force of attraction become less than that of expected.
Hence
the melting point of ‘Sb’ and ‘Bi’
elements is less than ‘As’ element.
·
Why is TlCl more stable than TlCl3 molecule?
·
Why is PbO2 act as a strong
oxidizing substance ?
·
Why Po(VI) compound act as a strong oxidizing substance
?
·
Why
is the melting point of ‘Sb’
and ‘Bi’ elements less than ‘As’ element ?








No comments