Xenon fluorides-xenon fluorides act as a strong oxidizing agent
Why are xenon fluorides act as a strong oxidizing agent ?
The
ionization potential of noble gas elements are very much high due to their complete valence
shell.
That
is why, inert gas elements have no tendency to participate in chemical reactions.
But,
under certain condition ‘Xe’ and Kr form
few fluorides as well as oxide
compounds.
So,
xenon fluorides have an affinity for electrons to regain inert gas configuration and
hence, xenon fluorides act as very
strong oxidizing reagent.
The
oxidizing power of xenon fluoride compounds
increase with increase in oxidation number of xenon(Xe) element.
For example,
xenon hexa-fluoride[ XeF6 ] is more stronger oxidizing than xenon
di-fluoride[XeF2 ].
There are
some chemical reaction of xenon fluorides where xenon fluorides act as oxidizing substances .
Such
as,xenon fluoride oxidized hydrogen
to form HF molecule and Cl–,
I– to Cl2 and I2 respectively.
It also
oxidized Ce(III) compounds to Ce(IV)
compounds and Br(V) compound to Br(VII ) compound.
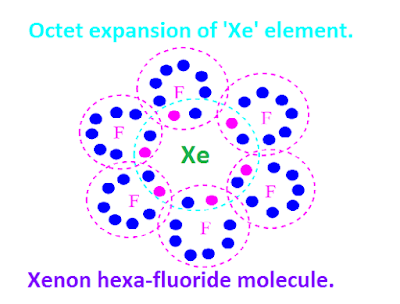
Octet expansion of xenon fluorides.
Xenon
fluorides and also other xenon compounds does not obey octet rule.
All
the xenon compounds including xenon fluorides show expansion of octet.
Summary :
Why are xenon fluorides act as a strong oxidizing substance ?
Octet expansion of xenon fluorides.


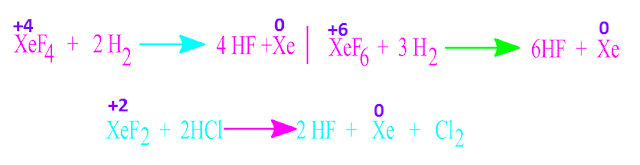







No comments