Lattice energy of MgF2-CaF2-ZrO2 molecule-explanation-calculation
·
Which one
has higher lattice energy, MgF2, CaF2 or ZrO2
?
Lattice energy is directlyproportional to the multiple of ionic charge and inversely proportional to the radius of cation and anion.
In the above
compounds, MgF2, CaF2 or ZrO2 , the extent of charge on‘Mg’ ,’Ca’ and
‘Zr’ metal are, +2 ,+2 and +4 respectively.
Again, the extent of charge on ‘F’ and ‘O’ atoms are -1 and -2 respectively.
So, from the data of cationic and anionic charge, it is clear that
the multiple of ionic charge for ZrO2 is highest.
Hence, ZrO2 molecule has higher lattice energy
among all the above compounds.
The value of lattice energy of MgF2, CaF2and ZrO2 molecules are, -2913 Kj/mole , -2609 Kj/mole
and - 8714.5 kJ/ mole respectively.
·
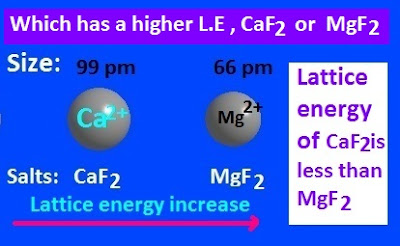
Which
one has higher lattice energy, MgF2
or CaF2 ?
In case of MgF2
and CaF2 molecule, the extent
of charge on both ‘Mg’ and’ Ca’ metal is, +2 and the extent of
charge on ‘F atom is, -1 .
That is, both cations and anions contain same extent of charge.
Now, both calcium and magnesium are group-II alkaline earth metal. It has
been found that, the cationic size of group-II metal decreases from top to
bottom.
Therefore, the radius of Mg2+ cation is less than Ca2+
cation.
Since, the lattice energy is directly proportional to the
multiple of ionic charge
and inversely proportional to the radius of cation and
anion, hence MgF2 has higher
lattice energy than CaF2.
The value of lattice energy of MgF2 and CaF2molecules are, -2913 Kj/mole , and -2609 Kj/mole respectively.
·
Why is
the lattice energy of ZrO2 so high ?
Lattice
energy is directly
proportional to the multiple of ionic charge and inversely
proportional to the radius of cation and anion.
Now,
in case of ZrO2 molecule, the extent of charge on ‘Zr’ metal atom is very much
high, that is, +4 and hence the
radius of Zr4+ cation is small.
Metal
with high charge and small radius cause the high lattice energy.
The
lattice energy of ZrO2 molecule is about, –9714.5 Kj/mole.
· Lattice energy calculation for ZrO2 molecule
Lattice
energy of ZrO2 can be calculated indirectly by using Born-Haber cycle.
The atomic number of oxygen and zirconium are 8 and 40
respectively.
Hence, the electronic configuration of
‘O’ and ‘Zr’ are [He]2s2
2p4 and [Kr] 4d2 5s2 respectively.
The first and second electron affinities of oxygen atom is +141
kj/mole and – 770 kj/mole respectively. The bond dissociation energy of oxygen molecule is 498 kj/ mole.
The sublimation energy of Zr(s ) to Zr(g) change is 591 kj/mole and
first, second ,
third and forth ionization energy of Zr are about 640 kj/mole,
1270 kj/mole, 2218 kj/mole and 3412 kj/mole respectively.
Applying, the above data, we can calculate the lattice energy of ZrO2 molecule. ΔHf of ZrO2 molecule is –1080 kj/mole. From
ΔHf
= S + [ I.E1 + I.E2 + I.E3 + I.E4 ]
+ D + 2 x [ E.A1 + E.A2 ] + U
or, –1080
= 591 + [ 640 +1270 +2218 +3412] + 2 x [ +141–770] +U
or, –1080 =
591 + 7540 + 2 x (–629) + U
or, –1080
= 591 + 7540 – 1258 + U
Therefore, U = –591 – 7540 + 1258 – 1080 = – 8451
kj/mole.
That is, the lattice energy of ZrO2 is –8451 kj/mole.
·
Which
one has higher lattice energy, MgF2 or CaF2 ?
·
Why
is the lattice energy of ZrO2
so high ?
·
Lattice energy calculation for ZrO2
molecule
·
What is lattice energy ?
·
What is unit of lattice energy ?
·
How lattice energy affect boiling
point ?
·
How lattice energy influence melting
point ?











No comments