activating groups-explanation-list with application
Activating groups explanation with an example.
Activating groups are those groups which increase the density of electron of benzene ring due to their positive inductive effect and resonance effect.
Since
these type of groups increase the electron density of benzene ring,
that is, activate the benzene ring, hence they are called activator enhancer or activating group.
that is, activate the benzene ring, hence they are called activator enhancer or activating group.
So,
due to presence of activating group,
the benzene ring becomes more activate towards electrophilic substitution reaction.
For
example, phenol with – OH activatinggroup under goes electrophilic
substitution reaction more easily than
benzene.
The central atom of activating group which directly attached to the benzene ring, contain one or more lone pair of electron.
So,
due to their +R effect, they increase electron density basically, on
the ortho and para carbon atom in
the benzene ring. Hence,
activating groups are also called ortho and para directing groups.
Now,
due to presence of activating group,
the electrophile attack the benzene ring easily than unsubstituted benzene ring
to form a ‘σ ‘-
complex.
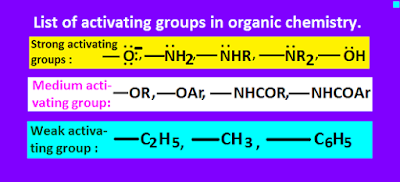
Activating groups list or examples.
There
are three types of activating groups,
namely, strong activating groups, medium activating groups and weak
activating groups.
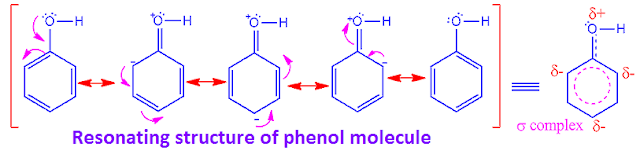
Why is phenol more active than benzene towards
electrophilic reaction ?
The –OH
group of phenol is an activating group. It increase the density of electron in
benzene ring due to its +R effect.
So, benzene
ring of phenol becomes more electron rich than benzene ring without any
activating group.
Hence,
phenol is more active than benzene towards electrophilic substitution reaction. The resonating structure of phenol molecule is shown above.
Why are halogens ortho and para directing group, in spite of deactivating in nature ?
Halogens are deactivating in nature . In
spite of this, they are ortho and para
directing group .
This can be
explained on the basis of two factors,
namely, inductive effect and mesomericeffect[ M effect ].
Halogen atom deactivate the benzene ring
due to its –I effect and activate
the benzene ring due to its +M or
resonance effect.
Now , in
case of halogen atoms , – I effect is
more effective than +M effect .So, halo
benzene should be inert in electro-philic
substitution reaction.
But in
practice, halo benzene undergoes electro-philicsubstitution reaction in ortho and para
position.
Because, due
to –I effect, halogens attract
electron charge from all position of benzene ring.
But, due to +M effect, it increase the electron density basically, on ortho and para carbon atom only.
Now , most
of the substitution reaction of benzene, halo benzene or other benzenoid compounds occurs by the attack of an electrophile.
Hence, the
substitution halo benzene occurs in ortho and para position only.

- Activating groups in organic chemistry ?
- Activating groups examples in chemistry.
- Why is phenol more active than benzene towards electrophilic reaction ?
- Why are halogens ortho and para directing group, in spite of deactivating in nature ?








No comments