Uses of hydrofluoric acid with health effect.
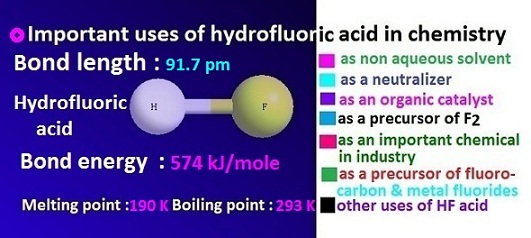
Important Uses of hydrofluoric acid in chemistry
Hydrofluoric
acid is
a concentrated aqueous solution of hydrogen chloride, with chemical formula,
HF. Density of hydrofluoric acid 1.15 g/c.c.
It is a polar
covalent compound. The dipole moment
of H–F bond is 1.86 D. Hydrofluoric acid
is a colorless, highly poisonous mono
basic weak acid.
The
melting and boiling point of hydrofluoric acid are, –83.6 ᵒC and 19.5 ᵒC respectively.
Although,
hydrofluoric acid is a poisonous
acid yet it has many chemical. Most amount of HF acid is used in industrialproduction.
Some
important uses of hydrofluoric acid in chemistry is discussed below.
Use of hydrofluoric acid as non aqueous solvent
Hydrofluoric
acid is one of the most water like solvents because, it is liquid at
temperatures below 19.5 ᵒC
upto –89.5 ᵒC.
The boiling
point of hydrofluoric acid is very high with respect to other non aqueous
solvent. Because of its high dielectric constant, it is used as a excellent
ionizing solvent.
But the use
of hydrofluoric acid as non aqueous solvent is limited due its high poisonous
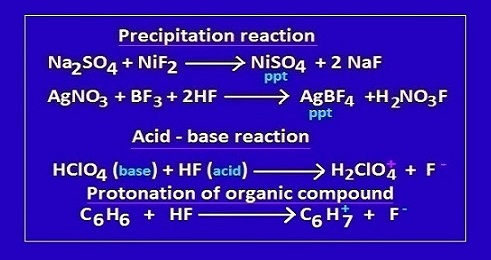
nature.Few important chemical reaction taking place in hydrofluoric acid medium
are,
Use of hydrofluoric acid as neutralizer
The high
value of specific conductance of hydrofluoric acid indicates that it under goes
auto ionization to H2F+ and HF2–ion.
Hence, any
substance which can yield H2F+ would behave as an acid
and any substance which produce HF2–or F– ion
would behave as a base.
There are
many acid-base reactions where hydrofluoric acid acts as a neutralizer.
For
example, in reaction with HNO3, H2SO4,
hydrofluoric acid behave as an acid and HNO3, H2SO4
act as base.
There are
another few acid-base reactions where hydrofluoric acid acts as a base and the
other participating substance act as Lewis base.
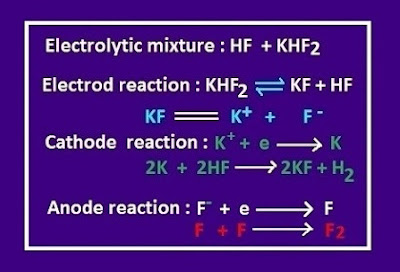
Use of hydrofluoric acid as a precursor of fluorine and metal fluorides
HF is the pioneer of elemental fluorine. F2 is produced by electrolysis
of a solution of HF and anhydrous KHF2.In this method a large amount
of F2 is prepared per every year.
HF
forms few addition compound with metal fluorides, such as, with KF, it form KHF2,
KF.2HF, KF.3HF and NH4F.HF etc.
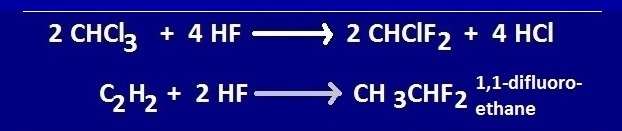
Use of hydrofluoric acid as a precursor of fluorocarbon
Hydrogen
fluoride is used to prepare fluorocarbons from chloro-carbons.An important
reaction of HF to form chloro carbon is
the production tetra fluoro ethylene or Teflon .
Again,
HF reacts with acetylene in the presence of ‘Hg’ catalyst and form
1,1-difluoroethane.
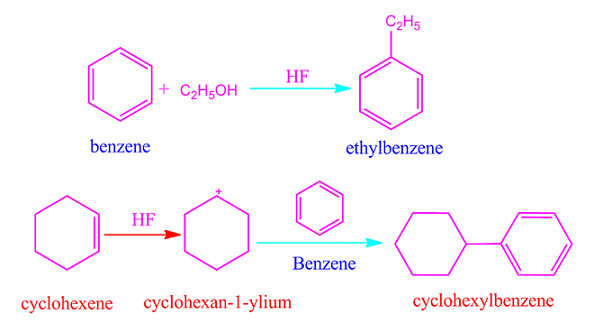
Use of hydrofluoric acid as an organic catalyst
HF acid is used as an organic catalyst. It is used in Friedel craft alkylation reaction to prepare ethyl benzene from benzene.
It is also
used in production of cyclohexylbenzene from cyclohexene.
Use of hydrofluoric acid as an important chemical in industry
About 60% of
entire hydrofluoric acid is used as different industrial purpose. For example, hydrofluoric acid is
used in production of refrigerants, herbicides,
pharmaceuticals,
gasoline, stainless steel kitchen products, plastics, electrical components
incandescent light bulbs etc.
Hydrofluoric acid is also used for etching glass
and enamel, removing rust, and cleaning brass and crystal in manufacturing
silicon semiconductor chips.
Others use of hydrofluoric acid
Due to its strong
corrosive qualities, a diluted form of hydrofluoric acid is used in some
commercial automotive cleaners, rust and stain removers and water-spot
removers.
HF
is used in the production of artificial cryolite [ AlF3. 3NaF ] from
which aluminium metal is extracted.
It is also used for separation of sand from
graphite and rote iron and analysis of silicate ores.
Health effects
Hydrogen fluoride is a poisonous gas. It has a bad impact on human organism.
When hydrogen fluoride come in contact with moisture,
including tissue, it immediately converts to hydrofluoric acid which is highly
corrosive and very toxic.
Hydrofluoric acid effected persons requires immediate
medical attention. Because, it can cause blindness due to rapid
destruction of corneas.
Besides, breathing in hydrogen fluoride at high levels or
in combination with skin contact can cause death.
- Important Uses of hydrofluoric acid in chemistry
- Use of hydrofluoric acid as non aqueous solvent
- Use of hydrofluoric acid as neutralizer
- Use of hydrofluoric acid as a precursor of fluorine and metal fluorides
- Use of hydrofluoric acid as an organic catalyst
- Use of hydrofluoric acid as an important chemical in industry









No comments