What is methane in organic chemistry?
What is methane in organic chemistry?
Methane is a hydride of carbon or hydrogen carbide. It is the simplest alkane of alkane family with the chemical formula CH4. Methane is also known as marsh gas, sweet gas, natural gas, dung gas.
The
main component of natural gas is methane.
It is a colorless, odorless, tasteless, flammable gas. Methane is a nonpolar covalent compound.
Hence it is slightly soluble in water but more soluble in organic solvents (alcohol, acetone, ether, benzene, toluene etc).
Methane is lighter than air. The vapour density of methane is half of the vapour density of air.
When cooled by applying pressure, methane first becomes liquid and then solidifies. The boiling point of methane is – 161.4ᵒC and the melting point is – 183 ᵒC.
The
density of methane is 0.66 kg / cubic meter at 298K temperature and 1
atmosphere pressure.
Read also : What is methyl alcohol in organic chemistry?
Methane chemical formula and structure
Methane is a covalent compound. It is a non polar compound and hence the dipole moment of methane is 0D.
From
primary analysis and vapor density found that the molecular formula of methane
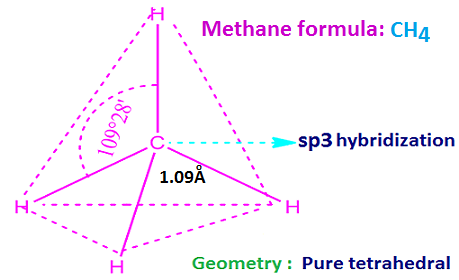
is CH4. That is, methane consists of one C-atom and four hydrogen atoms.
All the four hydrogen atoms of methane are equivalent. Because of substitution of one hydrogen atom by any univalent atom or group give only one mono substituted compound.
This implies that all four hydrogen atoms of methane are equivalent. The central carbon atom is sp3 hybridized.
So
the geometry of methane is pure tetrahedral. The H – C – H bond angle is 109ᵒ28’.
The C – H bond length is 1.09 Å.
Methane preparation
There are different methods for the preparation of methane, namely laboratory method, synthetic method and reduction method at room temperature.
Laboratory method:
In laboratory method, anhydrous sodium acetate is mixed with dry sodalime by 1:3 rations and then heated. As a result, impure methane is obtained.
These impurities are acetylene, ethylene, hydrogen, water vapor etc. After purification of these impurities, we can get pure methane.
Synthetic method:
In this method, a mixture of carbon monoxide or carbon dioxide and hydrogen is passed over a nickel dust at 300ᵒC - 400ᵒC temperature and as result of this methane is obtained.
Preparation of methane at room temperature:
At
room temperature, on reduction of methyl iodide by ethyl alcohol and Zn-Cu couple pure methane is obtained. Again, methane is also prepared by
hydrolysis of aluminium carbide.
Methane uses
Methane is the main component of CNG which is mainly used as fuel. It is a best fuel. Because of its combustion causes very low levels of air pollution.
In addition, its calorific value (5000 Btu per cubic foot) is much higher than other fuels.
For the above reason, methane is predominantly used as fuel. It is used as fuel in ovens, homes, water heaters, furnaces, cars, turbines and other things.
In many cities, methane is piped from house to house for domestic heating and cooking.
Again, refined liquid methane is mixed with liquid oxygen and used as rocket fuel.
As
a rocket fuel, methane offers the advantage over kerosene of producing small
exhaust molecules.
In addition, turbines or steam generators generate electricity using methane
gas as fuel.
In addition to fuel, methane has many other uses. For example, carbon
dioxide obtained from methane's heat dissipation is used to make paint,
printing inks and to make car tires.
Necessary
chemicals such as methyl chloride, acetylene, formaldehyde and methanol are
produced from methane.
The gas mixture (CO + H2) obtained by the reaction of steam with
methane is used in the production of methanol and hydrogen gas.
- What is methane in organic chemistry?
- Methane chemical formula and structure
- Methane uses
- Methane preparation
methane,
methane chemical formula, methane structure, methane uses, methane preparation,
Read more: What is hydroxylamine in organic chemistry?









Wonderful information thank you for nice knowledgeable topic about organic chemistry really,
ReplyDeleteHi,
ReplyDeleteI read your article and its so well written. I have also written something like yours. It would really be helpful if you could read my blog on Ear Surgery in kanpur and give your suggestions.
Thank You!