What is hydroxylamine?
What is hydroxylamine?
Hydroxylamine is a nitrogenous inorganic compound with chemical formula, NH2-OH. Hydroxylamine is also known as azinous acid, aminol, azanol, hydroxyazane, nitrinous acid etc.
When one hydrogen atom of ammonia is replaced by an –OH functional group, then the obtaining compound is called hydroxylamine.
There are different types of hydroxylamine, namely, methyl hydroxylamine, ethyl hydroxylamine, phenyl hydroxylamine etc.
Hydroxylamine is a colorless solid which melts at 306K temperature. Pure hydroxylamine is a white crystalline, hygroscopic compound.
It is unstable compound and decomposes violently even at 293K temperature to ammonia, nitrogen and water. The boiling point of hydroxylamine is 331K.
Hydroxylamine is freely soluble in water and lower
alcohols and the density of aqueous hydroxylamine solution is1.21 g/cc at 293K.
The aqueous solution of hydroxylamine is less basic than ammonia (Kb = 1.8 x 10-5).
NH2-OH (aq.) + H2O ⇌ NH3-OH+ + OH-, Kb = 6.6 x 10 -9.
The oxidation number of ‘N’-atom in hydroxylamine is
-1. Now the lowest and the highest oxidation number of ‘N’-atom are -3, +5
respectively.
Hence ‘N’-atom in hydroxylamine can increase its
oxidation number from -1 to +5 and decrease from -1 to -3.
Consequently hydroxylamine acts an oxidizing as well as
reducing agent depending upon circumstances.
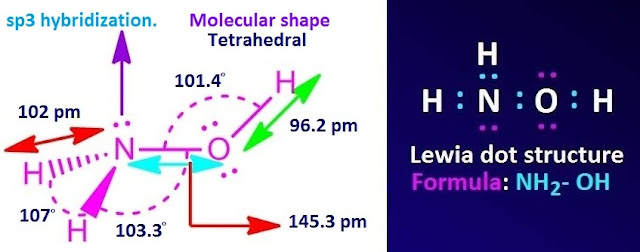
What is formula and Lewis dot structure of hydroxylamine?
Hydroxylamine or aminol is an inorganic compound with molecular formula, NH2OH. It is polar covalent compound.
The central nitrogen atom is directly attached with two hydrogen atoms and one oxygen atom. The nitrogen atom is sp3 hybridized.
So the co-ordination geometry of hydroxylamine is trigonal at ‘N’ and the molecular shape is tetrahedral at ‘N’.
Formula,
Lewis dot structure and molecular shape of hydroxylamine are shown below.
Hydroxylamine preparation:
Hydroxylamine is prepared by the reduction of nitrites
with SO2 under certain condition.
At first, a concentration solution of NaNO2 is mixed
with a solution of Na2CO3. Then SO2 is passed at a temperature below 276K, till
the solution becomes just acidic.
It is two steps process. In the first step, SO2 reacts
with sodium carbonate to give NaHSO3 and NaHCO3.
In the second step, NaHSO3 reacts with sodium nitrite to give hydroxylamine sodium sulphonate salt. This sulphonate salt can be easily hydrolysed to the expected hydroxylamine.
Hydroxylamine is also be prepared by the electrolytic
reduction of nitric acid in presence of 50% sulfuric acid, using amalgamated
‘Pb’-cathode.
What is phenyl hydroxylamine?
There are different types of hydroxylamine. Phenyl hydroxylamine is out of them. The chemical formula of phenyl hydroxylamine is C6H5-NH-OH.
When
one hydrogen atom (which attached with nitrogen atom) is substituted by phenyl
group (C6H5-), the obtaining hydroxylamine is known as phenyl hydroxylamine.
Is hydroxylamine acid or base?
The ‘N’-atom of hydroxylamine sp3 hybridized and it contains one lone pair of electron.
Hydroxylamine can donate this lone pair of electron to electron deficient species (atom, molecules or ions). So hydroxylamine acts as Lewis base.
The pKb value of hydroxylamine is 7.96. This means that, it is a weak base.
On the other hand, O – H bond in hydroxylamine is polar one due to electronegativity difference between oxygen and hydrogen elements.
Hence it can easily break and release H+ ion. Consequently, in this case hydroxylamine behaves as an acid.
The
pKa value of hydroxylamine is 6.02. This means that, it is a weak acid.
Hydroxylamine uses
Aqueous solutions of hydroxylamine are always used in chemical reactions. It is an important and effective reagent for the preparation of oximes. It is also an intermediate of biological nitrification.
Hydroxylamine and its salts are commonly used as reducing agents in countless organic and inorganic reactions. They can also act as antioxidants for fatty acids.
In the synthesis of Nylon 6, cyclohexanone is first converted to its oxime by condensation with hydroxylamine.
Hydroxylamine can also be used to highly selectively cleave asparaginyl-glycine peptide bonds in peptides and proteins.
It also bonds to and permanently disables heme-containing enzymes. It is used as an irreversible inhibitor of the oxygen-evolving complex of photosynthesis on account of its similar structure to water.
An alternative industrial synthesis of paracetamol developed by Hoechst–Celanese involves the conversion of ketone to a ketoxime with hydroxylamine.
Some non-chemical uses include removal of hair from animal hides and photographic developing solutions.
In
the semiconductor industry, hydroxylamine is often a component in the ‘resist
stripper’ which removes photoresist after lithography.
Hydroxylamine reaction with aldehyde and ketone
Hydroxylamine forms oximes with aldehyde and ketone. It forms aldoxime with aldehyde and ketoxime with ketone.
For example, hydroxylamine reacts with formaldehyde, acetaldehyde, benzaldehyde, acetone and forms formaldoxime, acetaldoxime, benzaldoxime and acetone oxime respectively.
What is the action of hydroxylamine on glucose?
Generally, hydroxylamine reacts with such compounds which contain aldehyde and keto groups.
Now glucose contains aldehyde group (-CHO). Hence hydroxylamine reacts with glucose and forms oxime, namely glucose oxime.
CH2-OH-(CH.OH)
4-CHO + NH2-OH ⟶ CH2-OH-(CH.OH)4-CH=N-OH + H2O
- What is hydroxylamine?
- What is formula and Lewis dot structure of hydroxylamine?
- Hydroxylamine preparation
- What is phenyl hydroxylamine?
- Is hydroxylamine acid or base?
- Hydroxylamine uses
- Hydroxylamine reaction with aldehyde and ketone
- What is the action of hydroxylamine on glucose?
Hydroxylamine,
hydroxylamine formula, hydroxylamine structure, NH2-OH, hydroxylamine
properties, hydroxylamine preparation, hydroxylamine uses,
Read more: What is ammonium nitrate?










No comments