What is hydrazine (NH2-NH2) gas?
What is hydrazine (NH2-NH2) gas?
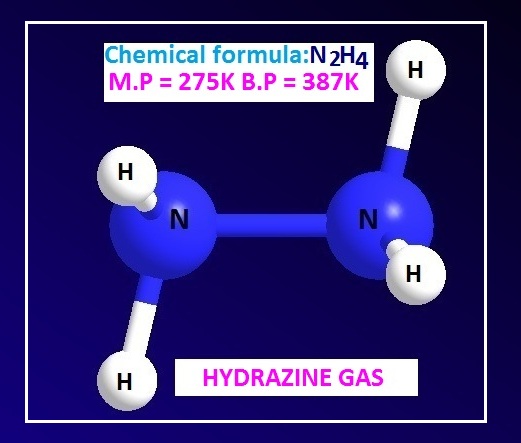
Hydrazine is an inorganic compound with chemical formula N2H4. It is a colorless fuming oily liquid with ammonia like odor. Hydrazine is strongly hygroscopic in nature.
The melting point of hydrazine is 275 K and the boiling point is 386.5 K. Hydrazine is soluble in water in all proportions. It is also soluble in alcohol.
The density of hydrazine is 1.02 g/cc. It is a polar compound and its dipole moment is 1.85 D. Hydrazine is a diacid base but exhibits both acidic and basic property.
Hydrazine preparation
Hydrazine is prepared by boiling a mixture of aqueous ammonia and alkaline solution of sodium hypochloride both in commercial as well as laboratory scale.
The reaction takes place in presence of a small amount of glue or gelatin. It is two steps process.
In the first step, ammonia reacts with NaOCl
giving chloramines, NH2-Cl. The chloramines then react with ammonia producing
hydrazine.
The overall reaction is as follows:
What is the formula and structure of hydrazine?
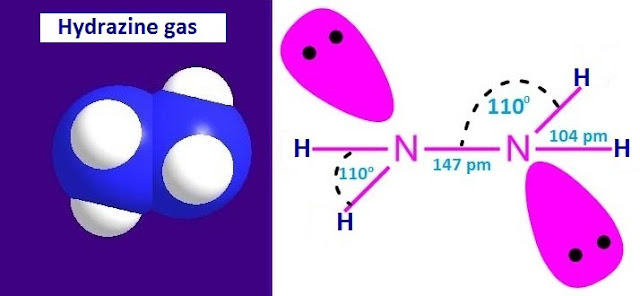
The molecular or chemical formula of hydrazine is N2H4 or H2N-NH2. The structure of hydrazine is very similar to the structure of H2O2.
Both H2O2 and N2H4 exist in gauche form at room temperature. The H –N –H bond angle is equal to the N –N – H bond angle.
The lone pairs of electrons on the two nitrogen atoms are trans to each other. The N –N bond distance and N –H bond distance are 1.47Å and 1.04 Å respectively.
In
hydrazine, both the nitrogen atoms are sp3 hybridized. So the molecular shape
of hydrazine is triangular pyramid at ‘N’.
Uses of hydrazine
· Hydrazine is very important reagent in organic as well as in inorganic chemistry.
In organic chemistry, it forms hydrazone with carbonyl compounds through which aldehyde and ketone compounds are identified.
· Since hydrazine and its salts can be readily oxidized to nitrogen, they are used as powerful reducing agents. Thus, hydrazine reduces salts of Pt, Au and Ag to the metallic state.
· Hydrazine and some of its derivatives are used as potential rocket fuels to prepare the gas precursors used in air bags.
· Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion.
· An important use of hydrazine in polymer chemistry as a foaming agent in preparing polymer foams.
·
In
polymer chemistry, its uses as a precursor to polymerization catalysts,
pharmaceuticals, and agrochemicals, as well as a long-term storable propellant
for in-space spacecraft propulsion.
What is the use of hydrazine fuel in space or car?
Hydrazine is used as a rocket fuel in space. This is because of hydrazine burns rapidly and completely in presence of air oxygen with the evolution of heat energy.
The reaction is exothermic and hence a lot of heat energy is obtained. The heat obtained is the driving force of rocket.
Sometimes, a mixture of hydrazine, methanol and water are used as rocket fuel in space. Hydrazine was first used as a component in rocket fuels during World War II.
Again, some derivatives of hydrazine are used as rocket fuel. For example, mono methyl hydrazine and unsymmetrical dimethyl hydrazine are used as rocket fuel.
These
derivatives are used in two-component rocket fuels, often together with N2O4.
These reactions are extremely exothermic, and the burning is also hypergolic.
Why is NH3 more basic than hydrazine (NH2-NH2)?
The nitrogen atom of ammonia and both the ‘N’-atom of hydrazine contain one lone pair of electron.
Both ammonia and hydrazine can donate their lone pair to electron deficit species. So both ammonia and hydrazine exhibit basic property.
But ammonia is more basic than hydrazine. Because of the strength of base depends on their conjugate acid.
Now the conjugate of NH3 and NH2-NH2 are ammonium ion (NH4+) and hydrazinium ion (NH2-NH3+) respectively.
It has been experimentally found that NH4+ ion is more stable than NH2-NH3+ ion which are supported by their dissociation constant.
The
Kb value for ammonia is 1.78 x 10 -5 while Kb value for hydrazine is 1.3 x 10
-6.
- What is hydrazine (NH2-NH2) gas?
- What is the formula and structure of hydrazine?
- Uses of hydrazine
- What is the use of hydrazine fuel in space or car?
- What makes hydrazine so dangerous?
- Why is NH3 more basic than hydrazine (NH2-NH2)?
hydrazine,
hydrazine gas, hydrazine formula, hydrazine structure, hydrazine uses,
hydrazine as rocket fuel,
Read more: What is hydroxylamine in chemistry?








No comments