What is magnesium carbonate?
What is magnesium carbonate?
Magnesium carbonate is an inorganic salt of carbonic acid with chemical formula MgCO3. Both anhydrous and hydrated forms of magnesium carbonate also exist as minerals.
The
most common forms of magnesium carbonate are anhydrous salt called magnesite
(MgCO3).
Read more: What is ethylene oxide or oxiran?
Some basic forms such as artinite (Mg2CO3 (OH)2·3H2O), hydromagnesite (Mg5(CO3)4(OH)2·4H2O), and dypingite (Mg5(CO3)4(OH)2·5H2O) also occur as minerals. All those minerals are colorless or white.
Magnesium carbonate chemical formula
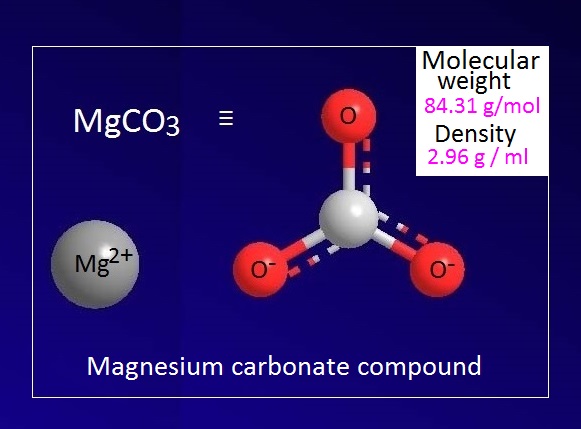
Magnesium carbonate has chemical formula of MgCO3. In fact, magnesium carbonate does not exist in the molecular state. Because of it is an ionic compound. Magnesium carbonate exists in the ionic form as Mg2+ CO32-.
The molecular or formula weight of anhydrous magnesium carbonate is 84.31 g/mol. Magnesium carbonate is a colorless odorless crystalline or white solid compound.
It
is hygroscopic in nature. Density of anhydrous magnesium carbonate is 2.96 g /
ml. The melting point of MgCO3 is 623K and it decomposes (anhydrous) at 438K
temperature.
Magnesium carbonate uses
Magnesium carbonate is an important
inorganic salt.
It is used as an essential ingredient in the preparation of
many important.
Magnesium carbonate is primarily used to prepare magnesium oxide by calcining method.
Minerals of magnesium carbonate (such as, magnesite and dolomite) are used to make refractory bricks.
MgCO3 is also used in flooring, fireproofing, fires extinguishing compositions, cosmetics, dusting powder and toothpaste.
Other applications are filler ingredients, plastic smoke suppressants, a strengthening agent in neoprene rubber, a drying agent and color retention in food.
Due to its low solubility in water and its hygroscopic properties, MgCO3 is added to the salt so that it flows more freely.
Magnesium carbonate, often referred to as "chalk", is also used as a drying agent by athletes in rock climbing, gymnastics, and weightlifting. It is also used as a food additive.
The only known side effect is that it can act as a high concentration laxative. Magnesium carbonate is used in taxidermis to whiten scalp.
It is mixed with hydrogen peroxide to form a paste. Magnesium carbonate is used as a matte white coating for projection screens.
Medical uses of magnesium carbonate
Magnesium
carbonate is non toxic and non-flammable. It is a laxative to loosen the
bowels. Highly-pure magnesium carbonate is used as an
antacid. In addition, it is used as an additive to table salt to keep it
flowing freely.
Is magnesium carbonate soluble in water?
Magnesium carbonate is an ionic compound. But it is partially soluble in polar water solvent. The solubility of magnesium carbonate in water is low because its solvation enthalpy is lower than that of lattice enthalpy.
The solubility of magnesium carbonate in water decreases with increasing temperature. The solubility of anhydrous MgCO3 in water at 298K is 0.0139 g/100 ml and the solubility at 373K is 0.0063/100 ml.
Magnesium carbonate is soluble in acid, aqueous carbon dioxide, and insoluble in acetone, ammonia.
MgCO3 + HCl Reaction example
Magnesium carbonate reacts with aqueous hydrochloric acid producing magnesium chloride, carbon dioxide and water.
MgCO3 (s) + 2HCl (dil) → MgCl2 + CO2 (g) + H2O (l)
MgCO3 + HNO3 Reaction example
Magnesium carbonate reacts with dilute solution of nitric acid producing magnesium nitrate, carbon dioxide and water.
MgCO3
(s) + 2HNO3 (dil) → Mg (NO3)2 + CO2 (g) + H2O (l)
Mgco3
chemical name, mgco3 chemical formula, molecular weight, molar mass, mgco3
properties, mgco3uses, MgCO3 + HCl, MgCO3 + HNO3
- What is magnesium carbonate?
- Magnesium carbonate chemical formula
- Magnesium carbonate uses
- Is magnesium carbonate soluble in water?
- MgCO3 + HCl
- MgCO3 + HNO3








No comments