What is exchange energy of electron in chemistry?
What is exchange energy of electron in chemistry?
When two electrons with
unidirectional spin in space exchange their position to each other, then the
actual arrangement of electron remain same.
But a small amount of energy of that electronic arrangement
decreases due to their exchange of position. As a result, the stability of the
electronic arrangement increases.
The amount of energy decreases for such type of position exchangebetween two electrons for one time is called exchange energy. Exchange energy is
expressed by the symbol ‘E'.
Example of exchange energy with p3 electronic configuration of a
molecule is shown
below. Exchange
energy explains why the half filled orbitals are exceptionally stable.
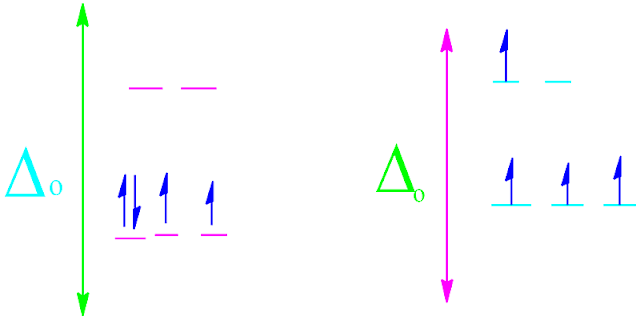
What is pairing energy in chemistry?
Electrons enter into nuclear orbit according to Hund's
rule. The arrangement of electrons inside atomic orbital occurs in such a way
that they minimize total energy.
When
an electron can singly occupy a given orbital in a para magnetic state
that configuration results in the high spin energy.
However,
when two electrons are force to occupy the same orbital, they experience an
inter-electronic repulsion effect on each other.
The
amount of energy is required to spin paired the two electron against this
repulsive force, is called pairing energy. It is denoted by the
symbol Δo.
Example
of pairing energy with d4 electronic configuration of a molecule is shown
below.
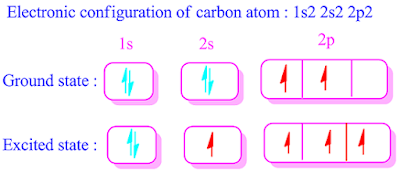
What is promotional energy,explain with example?
The
amount of energy is required for shifting of an electron from orbital of lower
energy to orbital of higher energy, is called promotional energy. The value of
promotional energy is positive.
Promotional
energy is expressed in term of "ΔE". Example of promotional energy of
a carbon atom is shown below.
The
ground state and excited state electronic configurations of carbon atom are
shown below respectively.
It has been found that, a electron from 2S orbital shifted to 2Pz orbital by absorbing energy in excited state. This is known as promotional energy.
Summary
- What is exchange energy of electron in chemistry?
- What is pairing energy, explain with example ?
- What is promotional energy, explain with example ?








Really helpful . Thanks a lot for posting this info
ReplyDeleteGreat explanation
ReplyDelete