Nucleophilic substitution reaction
Nucleophilic substitution reaction( SN1,SN2 ).
What is nucleophilic substitution reaction ?
When
one or more group or atom of a compound ( both aromatic and aliphatic ) is
replaced by the attact of another group or atom ( nucleophiles ), then this
reaction is called nucleophilic substitution reaction.
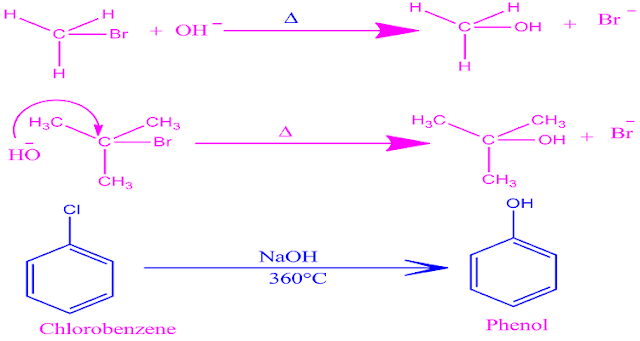
Example:
Nucleophiles: Nucleophiles are nucleus seeking group, molecules or ions that participate in nucleophilic substitution reaction .
Nucleophiles may be nutral molecule , anion , partially charged particle etc.Molecule with pi bond can also act as a nucleophile.
(I) Nutral nucleophile:
(II) Charged nucleophile:
Aliphatic nucleophilic substitution reaction:
When one or more group or atom of a compound ( both aromatic and
aliphatic ) is replaced by the attact of another group or atom ( nucleophiles ),
then this reaction is called nucleophilic substitution reaction.
There are two types of mechanism of nucleophilic
substitution reaction.
(I) Nucleophilic substitution unimolecular, denoted by SN1
A nucleophilic substitution
reaction on an sp3 carbon atom , in which only one species is involved in the
transition state of the rate determining step is known as nucleophilic substitution unimolecular.
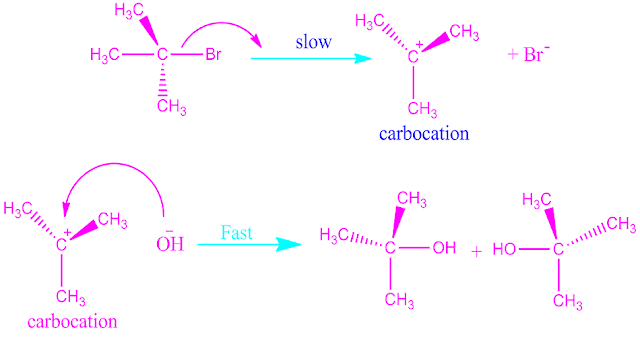
This is a two step
process. The first step consisting of slow heterolysis of the compound to form
a carbonium ion.
In the second step, rapidly combination between the
carbonium ion and the substituting nucleophile.
Since the rate
determining step is the first one, and since in this step ,only one
molecule is undergoing a covalency change,this type of mechanism
is called nucleophilic substitution unimolecular reaction and is labelled by
SN1.
step I : Slow dissociation of C―Br bond and formation of carbocation .
step II: Rapid combination between carbocation and substituting nucleophilic reagent .
The rate of this reaction depends on only concentration of substrate in the slow step. This is an example of first order reaction.
Rate of reaction = k[ t-butyl bromide]
The energy profile diagram of aliphatic nucleophilic substitution unimolecular reaction is shown
below.
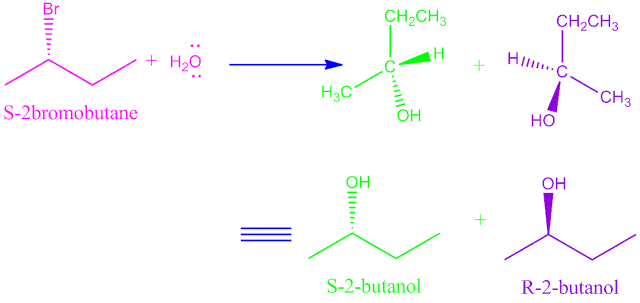
Stereo chemistry of SN1 reaction:
In SN1 reaction the leaving group leaves before the nucleophilic attact take place. this mean that,the nucleophile is free to attact either side of the planar trigonal carbo cation.
So two equal products of different configuration is obtained.
Because the SN1 mechanism is accompanied by inversion and
retension, but in some cases there may be complete inversion.
In SN1 mechanism the change in the hybridisation as well as geometry at saturated carbon from sp3→ sp2→sp3 and tetrahedral → trigonal→ tetrahedral.
Example:
Hydrolysis of ( S )-2-bromo butane.
(II) Nucleophilic substitution bimolecular, denoted by SN2.
It is an example of one step process. In the
transition state of this reaction on an sp3 carbon atom
involves two reacting species, so it is said to be nucleophilic substitution reaction bimolecular.
In an SN2 reaction breaking of bond between the leaving group and the carbon atom and making of bond between the carbon atom and the incoming nucleophile occurs simultaneously.
The transition state of an SN2 reaction involves both the substrate and the attacking nucleophile.
So the rate
of reaction depends on both the concentration of substrate and nucleophile. Hence
it is an example of second order reaction.
Rate of reaction = k[ substrate].[ nucleophile]
Example:
The energy profile diagram of aliphatic nucleophilic substitution reaction bimolecular( SN2) reaction is shown below.
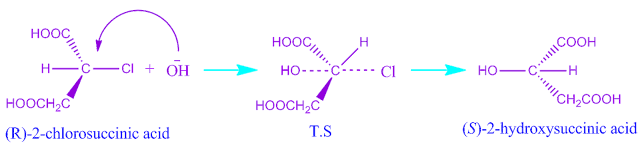
Stereo-chemistry of SN2 reaction:
During the SN2 reaction the incoming nucleophile attacts the substrate from back side.
So inversion of configuration of the product take place and it is called as walden inversion.
In SN2 reaction mechanism the hybridisation change from sp3→ sp2 , sp2→ sp3.
Example: Hydrolysis of (+) chloro succinic acid.
Nucleophilic aromatic substitution reaction:
Generally, there are three types of mechanism of aromatic nucleophilic substitution.
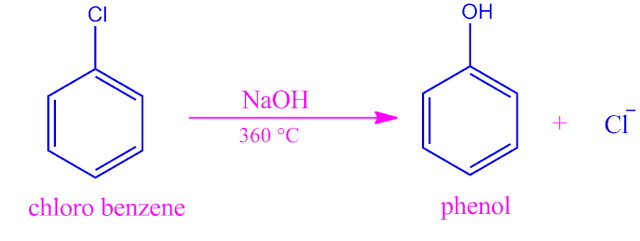
Example:substitution of chloro benzene by sodium hydroxide.
(I)Nucleophilic substitution unimolecular, denoted by SN1
The mechanism of aromatic nucleophilic sustitution unimolecular reaction is very rare.This reaction occurs in two step.
The first step being the slow step, it is the rate determining step. The reaction is found to follow the first order kenetics.
Rate of reaction = k[ substrate ]
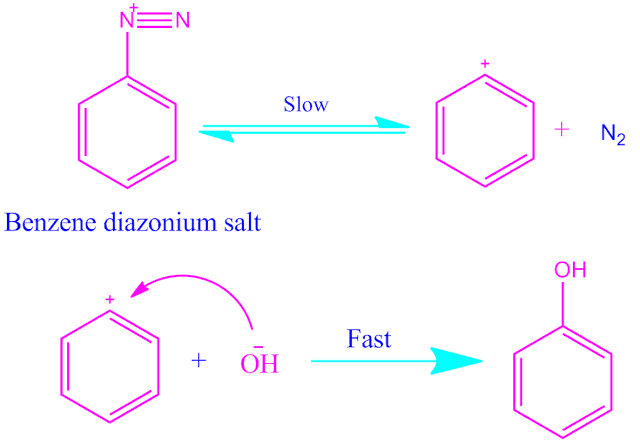
The most common example of this type of reaction is the displacement of N2 in the reaction of diazonium salts in strong polar medium.
step1: Slow ionisation of diazonium salts to form aryl cation reversively.
step2:Nucleophile attact on aryl cation very fast to form products.
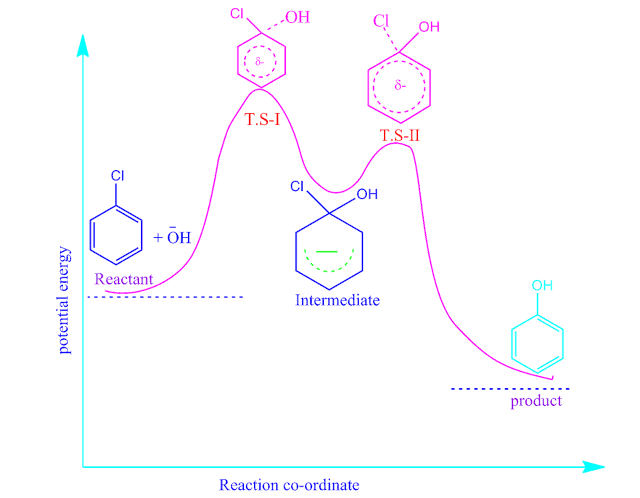
The energy profile diagram of nucleophilic aromatic substitution unimolecular reaction is shown below.
(II)Nucleophilic substitution bimolecular, denoted by SN2.
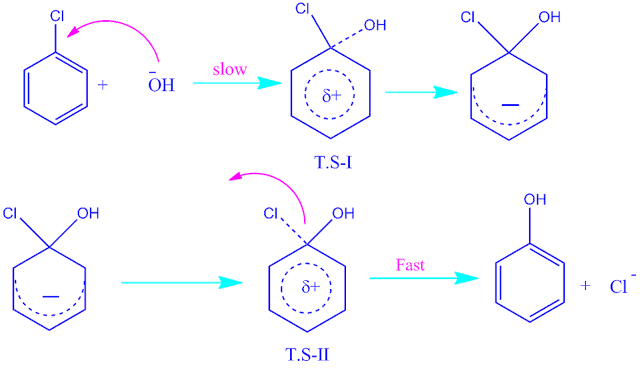
It is a two step process. In first step, nucleophile attact the aromatic compound and form a intermediate anion.
In the second step, loss of leaving group from the intermediate anion to yeild the products.
The first step being the slow step, it is the rate determining step. The reaction is found to follow the second order kenetics.
Because, the rate of this reaction depends on the initial concentration of both the participating molecules ( substrate, nucleophile ).
step1: In first step, nucleophile attact the chlorobenzene and form a intermediate anion.
step2:In the second step, loss of leaving group from the intermediate anion to yeild the products.
bimolecular reaction is given below.
(III)Addition-elimination reaction mechanism.
The reaction of ortho chloro nitobenzene and para chloro nitro benzene with base is an example of addition-elimination reaction.
Summary :
What is nucleophilic substitution reaction ?
Nucleophilic aromatic substitution reaction .
Addition-elimination reaction .
Stereochemistry of the nucleophilic substitution reaction.
















No comments