Formal charge, formal charge calculation and significance of formal charge
What is formal charge ?
Definition:
The charges placed on different atoms in the Lewis structure of a
molecule is called formal charge.
The formal charge may be defined as the charges which the atoms would
acquire in a molecule if all the atoms possessed the same
electronegativity.
Hence, it is assumed that the electron pairs in each bond are shared equally by the atoms involved.
Hence, it is assumed that the electron pairs in each bond are shared equally by the atoms involved.
example:
Formal charge calculation:
The formal charge on atoms in the Lewis structure may be calculated by
the following equation,
Significance of formal charge:
The assignment of formal charge to the different atoms in a Lewis
structure of molecule helps us to understand which structures are more stable
among the all probable structures.
Generally,the structure with smallest formal charges on the atoms is the most stable one.
For example, boron trifluoride may be exist in the following two form , (I) and (II) .
Placing lone pairs, the formal charges on the atoms in the two structures are calculated as,
From the above calculation, it is concluded that the structure (I) with smaller formal charge on the atoms would be more stable than structure ( II) .
Another example of cyanate ion . Cyanate ion has three Lewis structure, (I), (II) and (III).
But among this three, structurre(III) with smallest formal charge is more stable than the other two.
Another example of cyanate ion . Cyanate ion has three Lewis structure, (I), (II) and (III).
Formal charge of CO molecule in the Lewis structure .
Formal charge of ammonium cation ( NH4+ ) in the Lewis structure .
Practice problems .
what is formal charge ?
Calculate the formal charge of each atom in carbon dioxide molecule?
How the stability of a molecule or ion in Lewis structure depends on formal charge ?

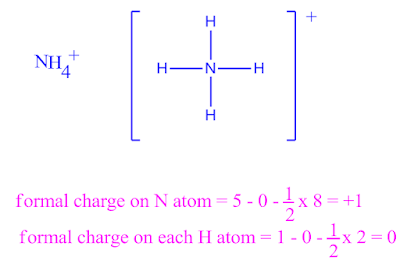












No comments