Triplet carbene is more stable than singlet carbene.
Why triplet carbene is more stable than singlet carbene?
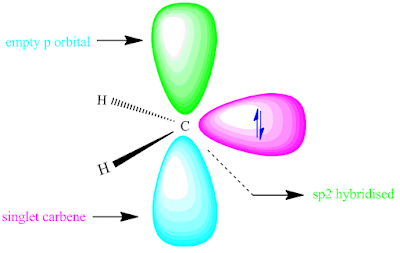
The stability of carbene depends on their electronic arrangement. Triplet carbene have two unpaired electrons in p orbital.
According to Hund`s rule, three multiplicity is possible. where as, the multiplicity of singlet carbene is one with a paired electron.
Besides, triplet carbene has 33 kj / mole energy which is lower than singlet carbene.
For the above two reason, triplet carbene is more stable than singlet carbene.
The structure of singlet carbene and triplet carbene is shown below.
Summary
- What is singlet carbene in chemistry?
- What is triplet carbene in organic chemistry?
- Why is triplet carbene more stable than singlet carbene?








No comments