Markownikoff’s-rule-definition-peroxide effect-limitation
Markownikoff’s rule in chemistry
When an addendum (halogen acids) is added to an asymmetric alkene, the negative part of the addendum is added on that double bonded carbon atom that contains least number of hydrogen atom.This statement is known as Markownikoff’s rule.
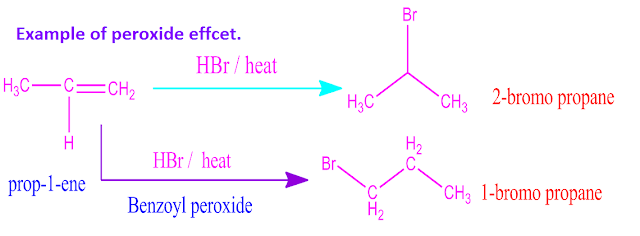
For example, addition of HBr to prop-1-ene gives 2-bromo propane.
Examples of
addendum : HCl , HBr , HI ,HCN H2SO4
, H2O etc molecules obey this rule.
What is peroxide effect in chemistry?
The addition of halogen acid and other addendum to an asymmetric alkene occur according to Markownikoff’s rule .
But ,if the
reaction is carried out in presence of oxygen ,ozone or any peroxide [ hydrogen
peroxide, benzoyl peroxide etc ] ,the addition of addendum to asymmetric
That is, the
negative part of the addendum is added on that double bonded carbon atom that
contain maximum number of hydrogen atom .
This fact of
opposite addition is known as peroxide effect .
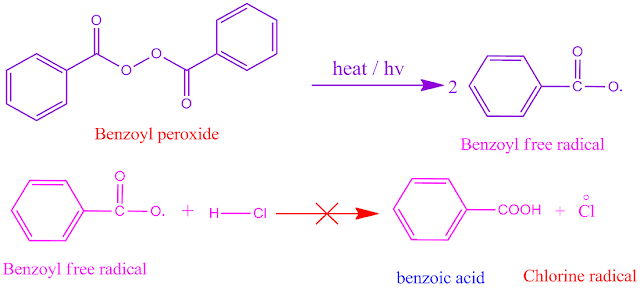
Why does HCl
molecule not show peroxide effect ?
The bond energy of HCl molecule is high ,that is, 103 Kcal / mol . In presence of peroxide, the reaction proceeds through free radical mechanism .
The benzoyl free radical from benzoyl peroxide is unabled to produce Clo free radical by the reaction with HCl molecule. Consequently , HCl does not response in peroxide effect .
Why does HI molecule not show peroxide effect ?
The bond energy of HI molecule is very low ( 71 Kcal /mol ) . In presence of peroxide , the reaction proceeds through free radical mechanism .
Hence, the
benzoyl free radical from benzoyl peroxide can produces ‘ Io’ free radical from HI molecule very easily.
Since, the
radius of ‘ Io’ free radical is large and hence two producing
‘ Io’ free radical unite to form iodine [ I 2
] molecule . Consequently
, H–I molecule does not show peroxide effect .
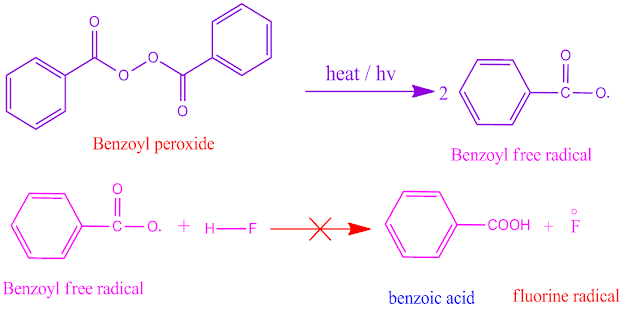
Why does HF molecule not show peroxide effect?
The bond energy of HF molecule is high ,that is , 136 Kcal / mol . In presence of peroxide, the reaction proceeds through free radical mechanism .
The benzoyl free radical from benzoyl peroxide is unabled to produce ‘Fo’ free radical by the reaction with HF molecule. Consequently , HF does not response in peroxide effect .
Summary
- Markownikoff’s rule in chemistry
- What is peroxide effect in chemistry
- Why does HCl molecule not show peroxide effect ?
- Why does HI molecule not show peroxide effect ?
- Why does HF molecule not show peroxide effect ?









No comments