What are the main causes of acid rain in environmental chemistry?
What are the main causes of acid rain in environmental chemistry?
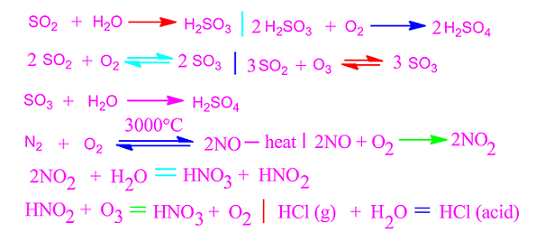
When some gaseous oxides and acid such as NO2,
SO2 and HCl etc are mixed with rain water and come down from the
atmosphere on the earth, then this phenomenon is called acid rain. There are two main causes of acid rain in environment.
These are,
·
Acid
rain due to natural causes and
·
Man-made causes of acid rain.
There are some natural events such as, ignition of
volcanoes, forest fire etc through which a tiny part of gaseous SO2,
NO2 etc entered into the air.
Besides, NO2 oxide is formed by the reaction
of atmospheric N2 and O2 at the time of lightning. NO2
is also formed due to the dissociation of nitrogenous compounds of soil by soil
bacteria and entered into the atmosphere.
Although, the above said natural causes enhance a
little portion of such type of non metallic acidic oxide into the atmosphere.
 |
| Volcanic eruption |
On the other hand, a huge extent of SO2, NO2
and HCl gas entered into the atmospheric air due to man-made causes.
The main man-made sources of SO2 and NO2
are thermal power station, acid production industry, motor vehicles, oil
refineries and many other sectors.
In the United States, about 70% of sulfur dioxide pollution
comes from power plants and about 40% of NO2 enter the atmosphere
from motor vehicles emitting with many other sources.
In Canada, about 60% of sulfur dioxide pollution comes from
industrial activities including oil refining and metal smelting.
Again, about 55%-60% of
NO2 gas enters into the air from motor vehicles emitting and many
other sources.
From the above contaminants sources, the gaseous
substances which are main causes of acid rain, enter the air.
These oxides directly combined with rain water and
produced different mineral acids such as sulfuric acid, nitric acid,hydrochloric acid etc and fall on the earth surface in the form of acid rain.
Again, these oxides are further oxidized by air O2,
O3 and other oxidizing substance, resulting in the formation of more
strong acid that creates acid rain.
Besides, from hydrogen chloride manufacturing industry,
a lot hydrogen chloride gas entered into the air. Now, HCl gas is a volatile
and highly water soluble substance.
So, it is readily dissolved in rain water and form hydrochloricacid that falls on the earth surface as acid rain.
What is the contribution of different acids in acid rain?
In the creation of acid rain, the contribution of
sulfuric acid is 62-65% and that of for nitric acid is 32-35%.
The other acids those are responsible for acid rainsare HCl, H2SO3 and HNO2 etc. The contribution
of HCl, H2SO3 and HNO2 acids are approximately
2%, 1-2% and 0-2% respectively.
- What are the main causes of acid rain in environmental chemistry?
- What is the contribution of different acids in acid rain?
- What is acid rain in environmental chemistry?










tuff
ReplyDelete