Why is Ni (CO) 4 tetrahedral and diamagnetic?
Why is Ni (CO) 4 tetrahedral and diamagnetic?
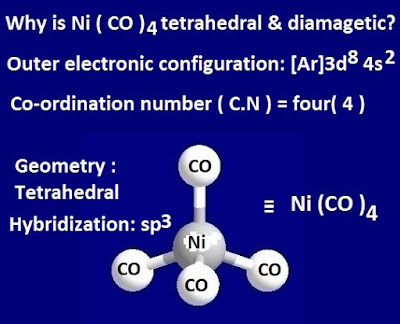
In the case of Ni (CO) 4 compound, the co-ordination number of Ni-metal is 4.
So the geometry of the Ni (CO) 4 compound may square
planar or tetrahedral depending on the hybridization of central metal Ni-atom.
If Ni-atom involves dsp2 hybridization, then the geometry of the compound is square planar.
Again, if it attains sp3 hybridization, then the geometry of the above nickel tetra carbonyl compound become tetrahedral.
Now the electronic configuration of nickel atom in
ground state is 3d8 4s2. But in excited state, the 4s electron of central meal
Ni-atom is shitted to 3d-sub shell.
Under this condition, the 3d sub shell occupied 10
electrons. That is, 3d-subshell has no unpaired electrons.
Now one vacant 4s orbital and three 4p orbital of outer
shell are mixed to each other and form energetically equivalent four hybridized
orbitals, involving sp3 hybridization.
These 4 vacant hybridized orbitals accept four-electron pair from four CO ligand forms L–M co-ordinate bond, resulting in the
formation of tetrahedral Ni (CO) 4 molecule.
According to Hund’s rule the electronic configuration
and hybridization of Ni (CO) 4 compound is shown below.
Now from the electronic configuration, it has been found that the 3d-orbital of central metal nickel atom has no unpaired electron.
Hence the tetrahedral Ni (CO) 4 compound diamagnetic in nature.
- Why is Ni (CO) 4 tetrahedral and diamagnetic?
- Why is the geometry of Ni (CO) 4 compound?
- Why is Ni (CO) 4 compound diamagnetic?
- Ni (CO) 4 compound tetrahedral and diamagnetic-explain why?
Ni (CO) 4 tetrahedral and diamagnetic, Nickel tetra
carbonyl diamagnetic, Ni (CO) 4 tetrahedral, Ni (CO) 4 compound diamagnetic,
Read more : Oxidation and reduction reactions with example









No comments