What is singlet and triplet carbene in chemistry?
What is singlet and triplet carbene in chemistry?
What is carbene in chemistry?
This is the first member of
alkenes. Carbene are very short lived species in which one carbon atom
possesses two bonds and two non bonded electrons, either paired or unpaired,
that is, carbene are called as a bivalent carbon compound.
For example, dichloro
carbene, diphenyl carbene etc.
There are two types of carbene.(I)
Singlet carbene (II) Triplet carbene. The preparation and application of these
types of carbene are discussed below.
What is Singlet carbene?
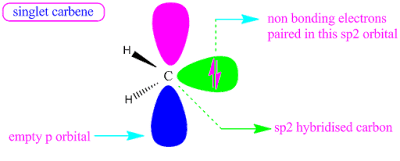
The two non bonded electrons of a carbene may be either paired or unpaired.When they are paired, then the carbene is called singlet carbene.
The two non bonded electrons of a carbene may be either paired or unpaired.When they are unpaired, then the carbene is called singlet carbene.
Preparation of carbene
(I) Carbenes are formed by the photochemical decomposition or pyrolysis of diazomethane or keten.
Reaction of carbene
Carbene shows different type of reactions,
such as, addition reaction, insertion, abstraction, rearrangement reaction and
dimerization reaction.
(II)Insertion reaction : Insertion into C一H bonds are as follows.
(IV)Dimerisation reaction
While this is often an unwanted side reaction, it can be
employed as a synthetic tool and a direct metal carbene dimerization has been
used in the synthesis of poly alkynyl ethenes.
Persistent carbene exist in equilibrium with their
respective dimer.
Carbene shows nucleophilic and chain
expansion reaction.
Electrophilic reaction of carbene : Reimer Tiemann reaction is an example of electrophilic reaction which followed by carbene.
Summary
- What is carbene, explain with example?
- What is singlet carbene? Give example.
- What is triplet carbene? Give example.
















No comments