Why Ni+2 paramagnetic whereas Zn+2 diamagnetic?
Why Ni+2 paramagnetic whereas Zn+2 diamagnetic?
Para magnetism and diamagnetism of a substance depends on the number of electrons occupied by it. A paramagnetic substance is one that contains one or more unpaired electrons.
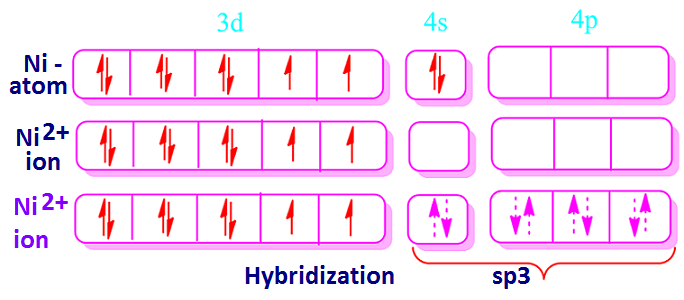
On the other hand, a diamagnetism substance is one that does not contain any odd electrons. Now, Ni +2 ion is 3d8 system. According to Hund’s rule the outer electronic configuration of Ni+2 ions is [Ar] 3d8.
From electronic configuration of Ni +2 ion, it has been found that it has two unpaired electrons.
Now, depending upon the hybridization, there are two types of possible structure of Ni +2 ion are formed with co-ordination number 4.
If
the complex involves ‘sp3’ hybridization, it would have tetrahedral structure.
Again, if the complex involves ‘dsp2’ hybridization, it would have squareplanar structure.
Consequently, for the formation of tetrahedral structure through the ‘sp3’ hybridization, the 3d-orbital of nickel atom remain unaffected.
Therefore,
3d-orbital of Ni +2 ion possessed two unpaired electrons and hence
the concern complex would be paramagnetic.
For
example, in case of [NiCl4] 2– complex ion, Ni+2 ions with co-ordination 4 involves ‘sp3’ hybridization. Hence the geometry of, [NiCl4] 2– complex ion would be tetrahedral.
Under
this condition, the electronic arrangement of
Ni +2 ion is evidently shown that the 3d-orbitals of Ni +2 ion have two unpaired electrons and hence Ni+2
ion is paramagnetic.
On the other hand the atomic number of zinc is 30. According to Hund’s rule the outer electronic configuration of Zn+2 ions is [Ar] 3d10 that is Zn+2 ions is 3d10 system.
That
is, 3d orbital of Zn+2 ions is completely fulfilled but outer 4s
orbital is vacant.
Hence
according to valence bond theory the outer ‘4s’ orbital of Zn+2 ion
is combined with the vacant three 4p
orbital and form energetically equivalent four sp3 hybridized orbitals.
Now,
four similar or dissimilar ligands are attached with these four hybridized
orbitals through the formation of four co-ordinate bond.
As
a result, Zn+2
ion
forms tetrahedral molecule involving ‘sp3’
hybridization.
For
example, in case of [Zn(NH3)4]+2 complex ion, Zn+2 ions
with co-ordination 4 involves ‘sp3’
hybridization. Hence the geometry of, [Zn(NH3)4]+2 complex
ion would be tetrahedral.
The outer electronic configuration of Zn+2 ions and its hybridization are shown below.
From
the above electronic configuration of Zn+2 ions, it has been found
that the number of unpaired electrons is zero. Since the Zn+2 ion
has no unpaired electrons, hence it is diamagnetic.
- Why Ni+2 paramagnetic whereas Zn+2 diamagnetic?
- Why is Ni+2 ion paramagnetic in nature?
- Why is Zn+2 ion diamagnetic in nature?
- Why Zn+2 diamagnetic whereas Ni+2 paramagnetic?
Ni+2 paramagnetic whereas Zn+2
diamagnetic, Zn+2 diamagnetic whereas Ni+2 paramagnetic, Ni+2
ion paramagnetic in nature, Zn+2 ion diamagnetic in nature,
Read more : Dehydrogenations of alcohol reactions










This type of hybrid orbital theory has no relevance to modern coordination chemistry - do not use it. It is not used in the chemical literature. Use CFT, or better MO theory.
ReplyDeletePakistan yurtdışı kargo
ReplyDeleteÖzbekistan yurtdışı kargo
Orta Afrika Cumhuriyeti yurtdışı kargo
Norveç yurtdışı kargo
Nikaraguay yurtdışı kargo
5GOHLO