What is dehydration synthesis reaction in organic chemistry?
What is dehydration synthesis reaction in organic chemistry?
The dehydration synthesis reaction is an important reaction in organic chemistry. This is because compounds like alkene, aldehyde, cyanide, ether, ester etc. are synthesized by this reaction.
The dehydration
synthesis reaction is the reverse of the hydration reaction. In the process of dehydration
synthesis, water molecules are removed from the reactant and produce products.
The chemical process by which water molecules are removed from one or more reactive molecules or ions to form a new substance is called a dehydration synthesis reaction.
Dehydration synthesis can be of two types, intra- molecular dehydration synthesis and intermolecular dehydration synthesis.
Common
dehydrating agents used in organic synthesis are concentrated sulfuric acid and
alumina. Also P2O5, iodine etc. can be used as
dehydrating agents.
What is an example of dehydration synthesis reaction?
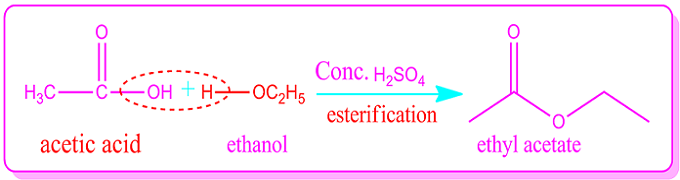
The best example of a dehydration synthesis reaction is the Fisher esterification reaction.
In this case esters are produced by the reaction of alcohol and carboxylic acids in the presence of dehydrating agents.
Using dehydration synthesis reaction two mono saccharides such as glucose and fructose are combined together to form disaccharide (saccharose).
There
are many examples of dehydration synthesis in organic synthesis, for example
dehydration of alcohol or sugars.
Also compounds like carboxylic acid, amide,
alcohol, aldol and aldoxim etc. take part in the dehydration synthesis
reaction.
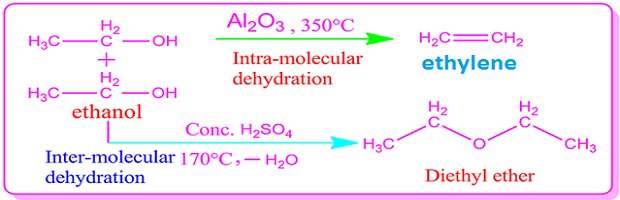
Alcohol
usually undergoes both intra molecular and intermolecular dehydration
synthesis.
Alkenes are formed in the intra molecular
dehydration of alcohol and ether in the intermolecular dehydration.
What is dehydration synthesis used for?
Ether and alkene are synthesized from alcohol through dehydration synthesis reaction. It commonly occurs in crating polymers, one example is the synthesis of glycogen from glucose.
In
addition, compounds like carboxylic acid and ester from alcohol, cyanide from
amide, acid anhydride from carboxylic acid and aldehyde also from carboxylic
acid are synthesized through dehydration synthesis reaction.
- What is dehydration synthesis reaction in organic chemistry?
- What is an example of dehydration synthesis reaction?
- What is dehydration synthesis used for?
- What is dehydration synthesis reaction?
Dehydration synthesis, dehydration synthesis
used for, dehydration synthesis reaction, dehydration synthesis simple
definition, dehydration synthesis example,
Read also : Dehydrogenation of alcohols in organic chemistry









No comments